Recycling of the cell adhesion molecule L1 in axonal growth cones
- PMID: 10804209
- PMCID: PMC1237010
- DOI: 10.1523/JNEUROSCI.20-10-03676.2000
Recycling of the cell adhesion molecule L1 in axonal growth cones
Abstract
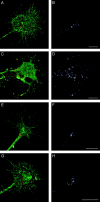
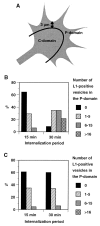
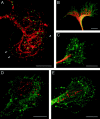
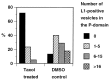
The cell adhesion molecule (CAM) L1 plays crucial roles in axon growth in vitro and in the formation of major axonal tracts in vivo. It is generally thought that CAMs link extracellular immobile ligands with retrogradely moving actin filaments to transmit force that pulls the growth cone forward. However, relatively little is known about the fate of CAMs that have been translocated into the central (C)-domain of the growth cone. We have shown previously that L1 is preferentially endocytosed at the C-domain. In the present study, we further analyze the subcellular distribution of endocytic organelles containing L1 at different time points and demonstrate that internalized L1 is transported into the peripheral (P)-domain of growth cones advancing via an L1-dependent mechanism. Internalized L1 is found in vesicles positioned along microtubules, and the centrifugal transport of these L1-containing vesicles is dependent on dynamic microtubules in the P-domain. Furthermore, we show that endocytosed L1 is reinserted into the plasma membrane at the leading edge of the P-domain. Monitoring recycled L1 reveals that it moves retrogradely on the cell surface into the C-domain. In contrast, the growth cone advancing independently of L1 internalizes and recycles L1 within the C-domain. For the growth cone to advance, the leading edge needs to establish strong adhesive interactions with the substrate while attachments at the rear are released. Recycling L1 from the C-domain to the leading edge provides an effective way to create asymmetric L1-mediated adhesion and therefore would be critical for L1-based growth cone motility.
Figures


Similar articles
-
The mechanism of axon growth: what we have learned from the cell adhesion molecule L1.Mol Neurobiol. 2003 Dec;28(3):219-28. doi: 10.1385/MN:28:3:219. Mol Neurobiol. 2003. PMID: 14709786 Review.
-
The role of endocytic l1 trafficking in polarized adhesion and migration of nerve growth cones.J Neurosci. 2001 Dec 1;21(23):9194-203. doi: 10.1523/JNEUROSCI.21-23-09194.2001. J Neurosci. 2001. PMID: 11717353 Free PMC article.
-
The neural cell adhesion molecule L1 interacts with the AP-2 adaptor and is endocytosed via the clathrin-mediated pathway.J Neurosci. 1998 Jul 15;18(14):5311-21. doi: 10.1523/JNEUROSCI.18-14-05311.1998. J Neurosci. 1998. PMID: 9651214 Free PMC article.
-
NGF enhances sensory axon growth induced by laminin but not by the L1 cell adhesion molecule.Mol Cell Neurosci. 2002 May;20(1):2-12. doi: 10.1006/mcne.2002.1107. Mol Cell Neurosci. 2002. PMID: 12056835
-
[Regulation of axonal growth cone motility by neural cell adhesion molecules: L1 intracellular trafficking and signaling pathways].Tanpakushitsu Kakusan Koso. 2000 Jul;45(10):1735-41. Tanpakushitsu Kakusan Koso. 2000. PMID: 10897686 Review. Japanese. No abstract available.
Cited by
-
The mechanism of axon growth: what we have learned from the cell adhesion molecule L1.Mol Neurobiol. 2003 Dec;28(3):219-28. doi: 10.1385/MN:28:3:219. Mol Neurobiol. 2003. PMID: 14709786 Review.
-
GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70.PLoS One. 2013 Nov 4;8(11):e79689. doi: 10.1371/journal.pone.0079689. eCollection 2013. PLoS One. 2013. PMID: 24223996 Free PMC article.
-
Mechanisms of polarized membrane trafficking in neurons -- focusing in on endosomes.Mol Cell Neurosci. 2011 Dec;48(4):278-87. doi: 10.1016/j.mcn.2011.06.013. Epub 2011 Jul 2. Mol Cell Neurosci. 2011. PMID: 21762782 Free PMC article. Review.
-
Flotillin-mediated endocytic events dictate cell type-specific responses to semaphorin 3A.J Neurosci. 2010 Nov 10;30(45):15317-29. doi: 10.1523/JNEUROSCI.1821-10.2010. J Neurosci. 2010. PMID: 21068336 Free PMC article.
-
TAG1 regulates the endocytic trafficking and signaling of the semaphorin3A receptor complex.J Neurosci. 2012 Jul 25;32(30):10370-82. doi: 10.1523/JNEUROSCI.5874-11.2012. J Neurosci. 2012. PMID: 22836270 Free PMC article.
References
-
- Archer F, Doherty P, Collins D, Bolsover S. CAMs and FGF cause a local submembrane calcium signal promoting axon outgrowth without a rise in bulk calcium concentration. Eur J Neurosci. 1999;11:3565–3573. - PubMed
-
- Bentley D, O'Connor TP. Cytoskeletal events in growth cone steering. Curr Opin Neurobiol. 1994;4:43–48. - PubMed
-
- Bretscher MS, Aguado-Velasco C. Membrane traffic during cell locomotion. Curr Opin Cell Biol. 1998;10:537–541. - PubMed
-
- Bridgman PC. Functional anatomy of the growth cone in relation to its role in locomotion and neurite assembly. In: Letourneau PC, Kater SB, Macagno ER, editors. The nerve growth cone. Raven; New York: 1992. pp. 39–53.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
