Olivocerebellar climbing fibers in the granuloprival cerebellum: morphological study of individual axonal projections in the X-irradiated rat
- PMID: 10804216
- PMCID: PMC6772707
- DOI: 10.1523/JNEUROSCI.20-10-03745.2000
Olivocerebellar climbing fibers in the granuloprival cerebellum: morphological study of individual axonal projections in the X-irradiated rat
Abstract
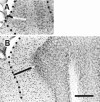
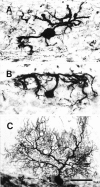
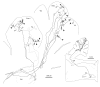
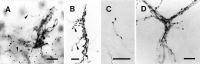
Elimination of cerebellar granule cells early during postnatal development produces abnormal neural organization that retains immature characteristics in the adult, including innervation of each Purkinje cell by multiple climbing fibers from the inferior olive. To elucidate mechanisms underlying development of the olivocerebellar projection, we studied light-microscopic morphology of single olivocerebellar axons labeled with biotinylated dextran amine in adult rats rendered agranular by a single postnatal X-irradiation. Each reconstructed olivocerebellar axon gave off approximately 12 climbing fibers, approximately twice as many as in normal rats. Terminal arborizations of climbing fibers made irregular tufts in most areas, whereas they were arranged vertically in a few mildly affected areas. Each climbing fiber terminal arborization innervated only part of the dendritic arbor of a Purkinje cell, and multiple climbing fibers innervated a single Purkinje cell. These climbing fibers originated either from the same olivocerebellar axon (pseudomultiple innervation) or from distinct axons (true multiple innervation). Abundant non-climbing fiber thin collaterals projected to all cortical layers. Although the longitudinal pattern of the zonal olivocerebellar projection was generally observed, lateral branching, including bilateral projections, was relatively frequent. These results suggest that the granule cell-parallel fiber system induces several important features of olivocerebellar projection: (1) organization of the climbing fiber terminal arborization tightly surrounding Purkinje cell dendrites, (2) elimination of pseudo- and true multiple innervations establishing one-to-one innervation, (3) retraction of non-climbing fiber thin collaterals from the molecular layer, and (4) probable refinement of the longitudinal projection domains by removing aberrant transverse branches.
Figures







Similar articles
-
Microzonal projection and climbing fiber remodeling in single olivocerebellar axons of newborn rats at postnatal days 4-7.J Comp Neurol. 2005 Jun 20;487(1):93-106. doi: 10.1002/cne.20531. J Comp Neurol. 2005. PMID: 15861456
-
Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat.J Comp Neurol. 1999 Nov 15;414(2):131-48. J Comp Neurol. 1999. PMID: 10516588
-
Organization and remodeling of the olivocerebellar climbing fiber projection.Cerebellum. 2006;5(1):15-22. doi: 10.1080/14734220500527385. Cerebellum. 2006. PMID: 16527759 Review.
-
Target-specific innervation of embryonic cerebellar transplants by regenerating olivocerebellar axons in the adult rat.Exp Neurol. 2002 Feb;173(2):205-12. doi: 10.1006/exnr.2001.7843. Exp Neurol. 2002. PMID: 11822884
-
Branching patterns of olivocerebellar axons in relation to the compartmental organization of the cerebellum.Front Neural Circuits. 2013 Feb 4;7:3. doi: 10.3389/fncir.2013.00003. eCollection 2013. Front Neural Circuits. 2013. PMID: 23382711 Free PMC article. Review.
Cited by
-
Architecture and development of olivocerebellar circuit topography.Front Neural Circuits. 2013 Jan 2;6:115. doi: 10.3389/fncir.2012.00115. eCollection 2012. Front Neural Circuits. 2013. PMID: 23293588 Free PMC article.
-
Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development.J Neurosci. 2001 Dec 15;21(24):9701-12. doi: 10.1523/JNEUROSCI.21-24-09701.2001. J Neurosci. 2001. PMID: 11739579 Free PMC article.
-
Genetic perturbation of postsynaptic activity regulates synapse elimination in developing cerebellum.Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16475-80. doi: 10.1073/pnas.0907298106. Epub 2009 Sep 4. Proc Natl Acad Sci U S A. 2009. PMID: 19805323 Free PMC article.
-
Multiple Phases of Climbing Fiber Synapse Elimination in the Developing Cerebellum.Cerebellum. 2018 Dec;17(6):722-734. doi: 10.1007/s12311-018-0964-z. Cerebellum. 2018. PMID: 30009357 Review.
-
The metamorphosis of the developing cerebellar microcircuit.Curr Opin Neurobiol. 2011 Apr;21(2):245-53. doi: 10.1016/j.conb.2011.01.009. Epub 2011 Feb 24. Curr Opin Neurobiol. 2011. PMID: 21353528 Free PMC article. Review.
References
-
- Altman J, Anderson WJ. Experimental reorganization of the cerebellar cortex. I. Morphological effects of elimination of all microneurons with prolonged X-irradiation started at birth. J Comp Neurol. 1972;146:355–406. - PubMed
-
- Altman J, Bayer SA. Development of the cerebellar system in relation to its evolution, structure, and functions. CRC; Boca Raton, FL: 1997.
-
- Bailly Y, Schoen SW, Delhaye-Bouchaud N, Kreutzberg GW, Mariani J. Synaptic localization of 5′-nucleotidase activity in the cerebellar cortex of the adult rat after postnatal X-irradiation. C R Acad Sci III. 1990;311:487–493. - PubMed
-
- Bailly Y, Schoen SW, Delhaye-Bouchaud N, Kreutzberg GW, Mariani J. 5′-nucleotidase activity as a synaptic marker of parasagittal compartmentation in the mouse cerebellum. J Neurocytol. 1995;24:879–890. - PubMed
-
- Bailly Y, Kyriakopoulou K, Delhaye-Bouchaud N, Mariani J, Karagogeos D. Cerebellar granule cell differentiation in mutant and X-irradiated rodents revealed by the neural adhesion molecule TAG-1. J Comp Neurol. 1996;369:150–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
