Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep
- PMID: 10804223
- PMCID: PMC6772663
- DOI: 10.1523/JNEUROSCI.20-10-03830.2000
Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep
Abstract
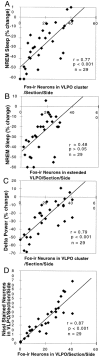
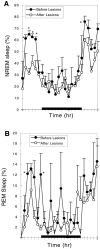
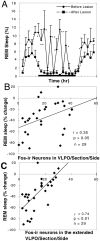
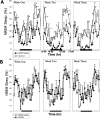
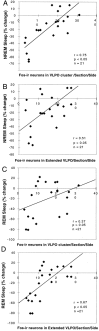
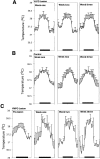
Neurons in the ventrolateral preoptic nucleus (VLPO) in rats show c-fos activation after sleep and provide GABAergic innervation of the major monoamine arousal systems, suggesting that they may be a necessary part of the brain circuitry that produces sleep. We examined the effects on sleep behavior in rats of cell-specific damage to the VLPO by microinjection of ibotenic acid. Severe lesions of the central cell cluster of the VLPO ( approximately 80-90% cell loss bilaterally) caused a 60-70% decrease in delta power and a 50-60% decrease in nonrapid-eye-movement (NREM) sleep time (p < 0.001). The number of remaining Fos-immunoreactive neurons in the VLPO cell cluster was linearly related to NREM sleep time (r = 0.77; p < 0.001) and total electroencephalogram delta power (r = 0. 79; p < 0.001) but not to rapid-eye-movement (REM) sleep (r = 0.35; p > 0.10). Lesions in the region containing scattered VLPO neurons medial or dorsal to the cell cluster caused smaller changes in NREM sleep time (24.5 or 15%, respectively) but were more closely associated with loss of REM sleep (r = 0.74; p < 0.01). The insomnia caused by bilateral VLPO lesions persisted for at least 3 weeks. Lesions of the VLPO caused no change in mean body temperature or its circadian variation; after small lesions of the ventromedial preoptic nucleus, body temperature showed normal circadian variation but a wider temperature range, and sleep behavior was not affected. These experiments delineate distinct preoptic sites with primary effects on the regulation of NREM sleep, REM sleep, and body temperature.
Figures



References
-
- Alam MN, McGinty D, Szymusiak R. Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am J Physiol. 1995;269:R1240–R1249. - PubMed
-
- Benington JH, Heller HC. REM-sleep timing is controlled homeostatically by accumulation of REM-sleep propensity in non-REM sleep. Am J Physiol. 1994;266:R1992–R2000. - PubMed
-
- Bjorkum AA, Ha QH, Saper CB. Afferents to and efferents from the raphe nuclei to the ventrolateral preoptic nucleus. Soc Neurosci Abstr. 1999;25:625.
-
- Gaus SE, Saper CB. Sleep-active neurons in the ventrolateral preoptic area (VLPO) are galaninergic. Soc Neurosci Abstr. 1999;25:625.
-
- John J, Kumar VM. The effects of NMDA lesion of the medial preoptic neurons on sleep and other functions. Sleep. 1998;21:587–598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
