Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin
- PMID: 10804232
- PMCID: PMC6772700
- DOI: 10.1523/JNEUROSCI.20-10-03926.2000
Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin
Abstract
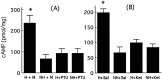
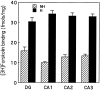
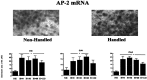
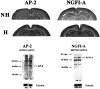
Postnatal handling increases glucocorticoid receptor expression in the rat hippocampus, thus altering the regulation of hypothalamic synthesis of corticotropin-releasing hormone and the hypothalamic-pituitary-adrenal response to stress. The effect on glucocorticoid receptor gene expression represents one mechanism by which the early environment can exert a long-term effect on neural development. The handling effect on hippocampal glucocorticoid receptor expression is dependent on peripheral thyroid hormone release and the activation of ascending serotonergic pathways. In primary hippocampal cell cultures, serotonin (5-HT) increases glucocorticoid receptor expression, and this effect appears to be mediated by increased cAMP levels. In the current studies we examined the in vivo effects of handling on hippocampal cAMP-protein kinase A (PKA) activity. In 7-d-old rat pups, we found that (1) postnatal handling increased adenylyl cyclase activity and hippocampal cAMP levels, (2) the effect of handling on cAMP levels was completely blocked by treatment with either propylthiouracil (PTU), a thyroid hormone synthesis inhibitor, or the 5-HT receptor antagonist, ketanserin, and (3) handling also increased hippocampal PKA activity. We then examined the effects of handling on cAMP-inducible transcription factors. Handling rapidly increased levels of the mRNAs for nerve growth factor-inducible factor A (NGFI-A) (zif268, krox24) and activator protein-2 (AP-2) as well as for NGFI-A and AP-2 immunoreactivity throughout the hippocampus. Finally, we found that the effects of handling on NGFI-A and AP-2 expression were significantly reduced by concurrent treatment with either PTU or ketanserin, effects that paralleled those on cAMP formation. NGFI-A and AP-2 have been implicated in the regulation of glucocorticoid receptor expression during development. Thus, these findings suggest that postnatal handling might alter glucocorticoid receptor gene expression via cAMP-PKA pathways involving the activation of NGFI-A and AP-2.
Figures





Similar articles
-
Ketanserin selectively blocks acute stress-induced changes in NGFI-A and mineralocorticoid receptor gene expression in hippocampal neurons.Neuroscience. 1997 Jan;76(2):441-8. doi: 10.1016/s0306-4522(96)00432-0. Neuroscience. 1997. PMID: 9015328
-
Effects of postischemic environment on transcription factor and serotonin receptor expression after permanent focal cortical ischemia in rats.Neuroscience. 2003;119(3):643-52. doi: 10.1016/s0306-4522(03)00195-7. Neuroscience. 2003. PMID: 12809685
-
Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade.Philos Trans R Soc Lond B Biol Sci. 2012 Sep 5;367(1601):2495-510. doi: 10.1098/rstb.2012.0223. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 22826348 Free PMC article.
-
Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus.Endocr J. 2009;56(3):335-44. doi: 10.1507/endocrj.k09e-075. Epub 2009 Apr 7. Endocr J. 2009. PMID: 19352056 Review.
-
Handling Continuous Outcomes in Quantitative Synthesis.2013 Jul 25. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008–. 2013 Jul 25. In: Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008–. PMID: 24006546 Free Books & Documents. Review. No abstract available.
Cited by
-
Role of AP-2alpha transcription factor in the regulation of synapsin II gene expression by dopamine D1 and D2 receptors.J Mol Neurosci. 2010 Jun;41(2):267-77. doi: 10.1007/s12031-009-9299-z. Epub 2009 Oct 20. J Mol Neurosci. 2010. PMID: 19842069
-
Glucocorticoids and fetal programming part 2: Mechanisms.Nat Rev Endocrinol. 2014 Jul;10(7):403-11. doi: 10.1038/nrendo.2014.74. Epub 2014 May 27. Nat Rev Endocrinol. 2014. PMID: 24863383 Review.
-
Early life adversity and the epigenetic programming of hypothalamic-pituitary-adrenal function.Dialogues Clin Neurosci. 2014 Sep;16(3):321-33. doi: 10.31887/DCNS.2014.16.3/canacker. Dialogues Clin Neurosci. 2014. PMID: 25364283 Free PMC article. Review.
-
Advances in TRH signaling.Rev Endocr Metab Disord. 2016 Dec;17(4):545-558. doi: 10.1007/s11154-016-9375-y. Rev Endocr Metab Disord. 2016. PMID: 27515033 Review.
-
Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids.J Physiol. 2006 Apr 1;572(Pt 1):31-44. doi: 10.1113/jphysiol.2006.105254. Epub 2006 Feb 9. J Physiol. 2006. PMID: 16469780 Free PMC article. Review.
References
-
- Bradford MM. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Biochemistry. 1976;72:248–254. - PubMed
-
- Brindley DN, Rolland Y. Possible connections between stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clin Sci. 1989;77:453–461. - PubMed
-
- de Kloet ER, Vregdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endo Rev. 1998;19:269–301. - PubMed
-
- Diorio J, Weaver SA, Sharma S, Chapman KE, Seckl JR, Meaney MJ. Handling increases hippocampal AP-2 and NGFI-A binding to a glucocorticoid receptor promoter oligonucleotide sequence. Soc Neurosci Abstr. 1997;23:1151.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
