Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid
- PMID: 10805722
- PMCID: PMC85698
- DOI: 10.1128/MCB.20.11.3781-3794.2000
Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid
Abstract
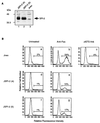
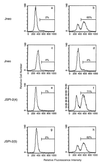
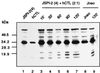
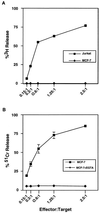
Cytotoxic T lymphocytes (CTL) can trigger an apoptotic signal through the Fas receptor or by the exocytosis of granzyme B and perforin. Caspase activation is an important component of both pathways. Granzyme B, a serine proteinase contained in granules, has been shown to proteolytically process and activate members of the caspase family in vitro. In order to gain an understanding of the contributions of caspases 8 and 3 during granule-induced apoptosis in intact cells, we have used target cells that either stably express the rabbitpox virus-encoded caspase inhibitor SPI-2 or are devoid of caspase 3. The overexpression of SPI-2 in target cells significantly inhibited DNA fragmentation, phosphatidylserine externalization, and mitochondrial disruption during Fas-mediated cell death. In contrast, SPI-2 expression in target cells provided no protection against granzyme-mediated apoptosis, mitochondrial collapse, or cytolysis, leading us to conclude that SPI-2-inhibited caspases are not an essential requirement for the granzyme pathway. Caspase 3-deficient MCF-7 cells were found to be resistant to CTL-mediated DNA fragmentation but not to CTL-mediated cytolysis and loss of the mitochondrial inner membrane potential. Furthermore, we demonstrate that granzyme B directly cleaves the proapoptotic molecule Bid, bypassing the need for caspase 8 activation of Bid. These results provide evidence for a two-pronged strategy for mediating target cell destruction and provide evidence of a direct link between granzyme B activity, Bid cleavage, and caspase 3 activation in whole cells.
Figures






References
-
- Atkinson E A, Barry M, Darmon A J, Shostak I, Turner P C, Moyer R W, Bleackley R C. Cytotoxic T lymphocyte-assisted suicide. J Biol Chem. 1998;273:21261–21266. - PubMed
-
- Atkinson E A, Bleackley R C. Mechanisms of lysis by cytotoxic T cells. Crit Rev Immunol. 1995;15:359–384. - PubMed
-
- Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1 and TNF receptor-induced cell death. Cell. 1996;85:803–815. - PubMed
-
- Caputo A, James M N G, Powers J C, Hudig D, Bleackley R C. Conversion of the substrate specificity of mouse proteinase granzyme B. Nat Struct Biol. 1994;1:364–367. - PubMed
-
- Caputo A, Parrish J C, James M N G, Powers J C, Bleackley R C. Electrostatic reversal of serine proteinase substrate specificity. Proteins. 1999;35:415–424. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
