Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae
- PMID: 10805747
- PMCID: PMC85775
- DOI: 10.1128/MCB.20.11.4049-4061.2000
Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae
Abstract
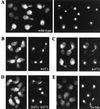
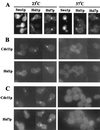
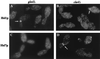
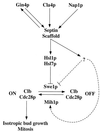
Saccharomyces cerevisiae septin mutants have pleiotropic defects, which include the formation of abnormally elongated buds. This bud morphology results at least in part from a cell cycle delay imposed by the Cdc28p-inhibitory kinase Swe1p. Mutations in three other genes (GIN4, encoding a kinase related to the Schizosaccharomyces pombe mitotic inducer Nim1p; CLA4, encoding a p21-activated kinase; and NAP1, encoding a Clb2p-interacting protein) also produce perturbations of septin organization associated with an Swe1p-dependent cell cycle delay. The effects of gin4, cla4, and nap1 mutations are additive, indicating that these proteins promote normal septin organization through pathways that are at least partially independent. In contrast, mutations affecting the other two Nim1p-related kinases in S. cerevisiae, Hsl1p and Kcc4p, produce no detectable effect on septin organization. However, deletion of HSL1, but not of KCC4, did produce a cell cycle delay under some conditions; this delay appears to reflect a direct role of Hsl1p in the regulation of Swe1p. As shown previously, Swe1p plays a central role in the morphogenesis checkpoint that delays the cell cycle in response to defects in bud formation. Swe1p is localized to the nucleus and to the daughter side of the mother bud neck prior to its degradation in G(2)/M phase. Both the neck localization of Swe1p and its degradation require Hsl1p and its binding partner Hsl7p, both of which colocalize with Swe1p at the daughter side of the neck. This localization is lost in mutants with perturbed septin organization, suggesting that the release of Hsl1p and Hsl7p from the neck may reduce their ability to inactivate Swe1p and thus contribute to the G(2) delay observed in such mutants. In contrast, treatments that perturb actin organization have little effect on Hsl1p and Hsl7p localization, suggesting that such treatments must stabilize Swe1p by another mechanism. The apparent dependence of Swe1p degradation on localization of the Hsl1p-Hsl7p-Swe1p module to a site that exists only in budded cells may constitute a mechanism for deactivating the morphogenesis checkpoint when it is no longer needed (i.e., after a bud has formed).
Figures






Similar articles
-
Roles of Hsl1p and Hsl7p in Swe1p degradation: beyond septin tethering.Eukaryot Cell. 2012 Dec;11(12):1496-502. doi: 10.1128/EC.00196-12. Epub 2012 Oct 5. Eukaryot Cell. 2012. PMID: 23042131 Free PMC article.
-
The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p.Mol Cell Biol. 1999 Oct;19(10):6929-39. doi: 10.1128/MCB.19.10.6929. Mol Cell Biol. 1999. PMID: 10490630 Free PMC article.
-
Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast.Genes Dev. 1999 Jan 15;13(2):176-87. doi: 10.1101/gad.13.2.176. Genes Dev. 1999. PMID: 9925642 Free PMC article.
-
Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint.Curr Opin Microbiol. 2006 Dec;9(6):540-6. doi: 10.1016/j.mib.2006.10.004. Epub 2006 Oct 19. Curr Opin Microbiol. 2006. PMID: 17055334 Review.
-
The morphogenesis checkpoint: how yeast cells watch their figures.Curr Opin Cell Biol. 2003 Dec;15(6):648-53. doi: 10.1016/j.ceb.2003.09.001. Curr Opin Cell Biol. 2003. PMID: 14644188 Review.
Cited by
-
Comprehensive Genetic Analysis of Paralogous Terminal Septin Subunits Shs1 and Cdc11 in Saccharomyces cerevisiae.Genetics. 2015 Jul;200(3):821-41. doi: 10.1534/genetics.115.176495. Epub 2015 May 12. Genetics. 2015. PMID: 25971665 Free PMC article.
-
Disappearance of the budding yeast Bub2-Bfa1 complex from the mother-bound spindle pole contributes to mitotic exit.J Cell Biol. 2006 Jan 30;172(3):335-46. doi: 10.1083/jcb.200507162. J Cell Biol. 2006. PMID: 16449187 Free PMC article.
-
Budding yeast PAK kinases regulate mitotic exit by two different mechanisms.J Cell Biol. 2003 Mar 17;160(6):857-74. doi: 10.1083/jcb.200209097. J Cell Biol. 2003. PMID: 12642613 Free PMC article.
-
The identification of Pcl1-interacting proteins that genetically interact with Cla4 may indicate a link between G1 progression and mitotic exit.Genetics. 2004 Mar;166(3):1177-86. doi: 10.1534/genetics.166.3.1177. Genetics. 2004. PMID: 15082539 Free PMC article.
-
The Sda1 protein is required for passage through start.Mol Biol Cell. 2001 Jan;12(1):201-19. doi: 10.1091/mbc.12.1.201. Mol Biol Cell. 2001. PMID: 11160833 Free PMC article.
References
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1995.
-
- Bagrodia S, Cerione R A. Pak to the future. Trends Cell Biol. 1999;9:350–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
