A green fluorescent protein-reporter mammalian two-hybrid system with extrachromosomal maintenance of a prey expression plasmid: application to interaction screening
- PMID: 10805780
- PMCID: PMC25809
- DOI: 10.1073/pnas.97.10.5220
A green fluorescent protein-reporter mammalian two-hybrid system with extrachromosomal maintenance of a prey expression plasmid: application to interaction screening
Abstract
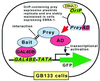
An improved mammalian two-hybrid system designed for interaction trap screening is described in this paper. CV-1/EBNA-1 monkey kidney epithelial cells expressing Epstein-Barr virus nuclear antigen 1 (EBNA-1) were stably transfected with a reporter plasmid for GAL4-dependent expression of the green fluorescent protein (GFP). A resulting clone, GB133, expressed GFP strongly when transfected transiently with transcriptional activators fused to GAL4 DNA-binding domain with minimal background GFP expression. GB133 cells maintained plasmids containing the OriP Epstein-Barr virus replication origin that directs replication of plasmids in mammalian cells in the presence of the EBNA-1 protein. GB133 cells transfected stably with a model bait expressed GFP when further transfected transiently with an expression plasmid for a known positive prey. When the bait-expressing GB133 cells were transfected transiently with an OriP-containing expression plasmid for the positive prey together with excess amounts of empty vector, cells that received the positive prey were readily identified by green fluorescence in cell culture and eventually formed green fluorescent microcolonies, because the prey plasmid was maintained by the EBNA-1/Ori-P system. The green fluorescent microcolonies were harvested directly from the culture dishes under a fluorescence microscope, and total DNA was then prepared. Prey-encoding cDNA was recovered by PCR using primers annealing to the vector sequences flanking the insert-cloning site. This system should be useful in mammalian cells for efficient screening of cDNA libraries by two-hybrid interaction.
Figures


Similar articles
-
Epi-CHO, an episomal expression system for recombinant protein production in CHO cells.Biotechnol Bioeng. 2005 Sep 20;91(6):670-7. doi: 10.1002/bit.20534. Biotechnol Bioeng. 2005. PMID: 15948170
-
Human p32: a coactivator for Epstein-Barr virus nuclear antigen-1-mediated transcriptional activation and possible role in viral latent cycle DNA replication.Virology. 2000 Sep 15;275(1):145-57. doi: 10.1006/viro.2000.0508. Virology. 2000. PMID: 11017796
-
High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells.Nucleic Acids Res. 2002 Jan 15;30(2):E9. doi: 10.1093/nar/30.2.e9. Nucleic Acids Res. 2002. PMID: 11788735 Free PMC article.
-
The uses of green fluorescent protein in mammalian cells.Methods Biochem Anal. 2006;47:305-37. doi: 10.1002/0471739499.ch14. Methods Biochem Anal. 2006. PMID: 16335719 Review. No abstract available.
-
Green fluorescent proteins light the way to a better understanding of the function and regulation of specific anterior pituitary cells.Endocrinology. 2000 Dec;141(12):4331-3. doi: 10.1210/endo.141.12.7939. Endocrinology. 2000. PMID: 11108239 Review. No abstract available.
Cited by
-
Network medicine in Cardiovascular Research.Cardiovasc Res. 2021 Aug 29;117(10):2186-2202. doi: 10.1093/cvr/cvaa321. Cardiovasc Res. 2021. PMID: 33165538 Free PMC article. Review.
-
Heteromeric MAPPIT: a novel strategy to study modification-dependent protein-protein interactions in mammalian cells.Nucleic Acids Res. 2003 Jul 15;31(14):e75. doi: 10.1093/nar/gng075. Nucleic Acids Res. 2003. PMID: 12853652 Free PMC article.
-
The latency-associated nuclear antigen, a multifunctional protein central to Kaposi's sarcoma-associated herpesvirus latency.Future Microbiol. 2011 Dec;6(12):1399-413. doi: 10.2217/fmb.11.137. Future Microbiol. 2011. PMID: 22122438 Free PMC article. Review.
-
Endogenous activation of glucokinase by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase is glucose dependent.Mol Endocrinol. 2010 Oct;24(10):1988-97. doi: 10.1210/me.2010-0115. Epub 2010 Aug 11. Mol Endocrinol. 2010. PMID: 20702580 Free PMC article.
-
Using an aplysia two-hybrid system to examine the interactions between transcription factors involved in long-term facilitation in the nervous system of aplysia.Learn Mem. 2003 Jan-Feb;10(1):40-3. doi: 10.1101/lm.55303. Learn Mem. 2003. PMID: 12551962 Free PMC article.
References
-
- Allen J, Walberg M, Edwards M, Elledge S. Trends Biochem Sci. 1995;20:511–516. - PubMed
-
- Brachmann R, Boeke J. Curr Opin Biotechnol. 1997;8:561–568. - PubMed
-
- Brent R, Finley R J. Annu Rev Genet. 1997;31:663–704. - PubMed
-
- Young K. Biol Reprod. 1998;58:302–311. - PubMed
-
- Frederickson R. Curr Opin Biotechnol. 1998;9:90–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

