MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration
- PMID: 10805784
- PMCID: PMC25813
- DOI: 10.1073/pnas.97.10.5243
MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration
Abstract
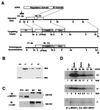
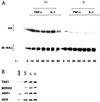
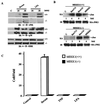
Exposure of eukaryotic cells to extracellular stimuli results in activation of mitogen-activated protein kinase (MAPK) cascades composed of MAPKs, MAPK kinases (MAP2Ks), and MAPK kinase kinases (MAP3Ks). Mammals possess a large number of MAP3Ks, many of which can activate the c-Jun N-terminal kinase (JNK) MAPK cascade when overexpressed, but whose biological function is poorly understood. We examined the function of the MAP3K MEK kinase 1 (MEKK1) in proinflammatory signaling. Using MEKK1-deficient embryonic stem cells prepared by gene targeting, we find that, in addition to its function in JNK activation by growth factors, MEKK1 is required for JNK activation by diverse proinflammatory stimuli, including tumor necrosis factor alpha, IL-1, double-stranded RNA, and lipopolysaccharide. MEKK1 is also essential for induction of embryonic stem cell migration by serum factors, but is not required for activation of other MAPKs or the IkappaB kinase signaling cascade.
Figures


References
-
- Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. Science. 1993;260:315–319. - PubMed
-
- Minden A, Lin A, McMahon M, Lange-Carter C, Dérijard B, Davis R J, Johnson G L, Karin M. Science. 1994;266:1719–1723. - PubMed
-
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Genes Dev. 1993;7:2135–2148. - PubMed
-
- Dérijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

