Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity
- PMID: 10805796
- PMCID: PMC25833
- DOI: 10.1073/pnas.090013497
Chromosomal breakage-fusion-bridge events cause genetic intratumor heterogeneity
Abstract
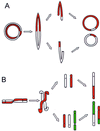
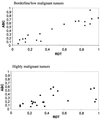
It has long been known that rearrangements of chromosomes through breakage-fusion-bridge (BFB) cycles may cause variability of phenotypic and genetic traits within a cell population. Because intercellular heterogeneity is often found in neoplastic tissues, we investigated the occurrence of BFB events in human solid tumors. Evidence of frequent BFB events was found in malignancies that showed unspecific chromosome aberrations, including ring chromosomes, dicentric chromosomes, and telomeric associations, as well as extensive intratumor heterogeneity in the pattern of structural changes but not in tumors with tumor-specific aberrations and low variability. Fluorescence in situ hybridization analysis demonstrated that chromosomes participating in anaphase bridge formation were involved in a significantly higher number of structural aberrations than other chromosomes. Tumors with BFB events showed a decreased elimination rate of unstable chromosome aberrations after irradiation compared with normal cells and other tumor cells. This result suggests that a combination of mitotically unstable chromosomes and an elevated tolerance to chromosomal damage leads to constant genomic reorganization in many malignancies, thereby providing a flexible genetic system for clonal evolution and progression.
Figures



References
-
- Örndal C, Rydholm A, Willén H, Mitelman F, Mandahl N. Cancer Genet Cytogenet. 1994;78:127–137. - PubMed
-
- Griffin C A, Hruban R H, Morsberger L A, Ellingham T, Long P P, Jaffee E M, Hauda K M, Bohlander S K, Yeo C J. Cancer Res. 1995;55:2394–2399. - PubMed
-
- Gorunova L, Johansson B, Dawiskiba S, Andrén-Sandberg A, Jin Y, Mandahl N, Heim S, Mitelman F. Genes Chromosomes Cancer. 1995;14:259–266. - PubMed
-
- Gorunova L, Höglund M, Andrén-Sandberg A, Dawiskiba S, Jin Y, Mitelman F, Johansson B. Genes Chromosomes Cancer. 1998;23:81–99. - PubMed
-
- Roberts C G, Tattersall M H. Cancer Genet Cytogenet. 1990;48:243–253. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

