Accumulation of protease-resistant prion protein (PrP) and apoptosis of cerebellar granule cells in transgenic mice expressing a PrP insertional mutation
- PMID: 10805813
- PMCID: PMC25870
- DOI: 10.1073/pnas.97.10.5574
Accumulation of protease-resistant prion protein (PrP) and apoptosis of cerebellar granule cells in transgenic mice expressing a PrP insertional mutation
Abstract
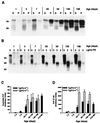
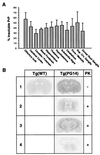
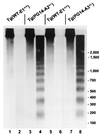
We have generated lines of transgenic mice that express a mutant prion protein (PrP) containing 14 octapeptide repeats whose human homologue is associated with an inherited prion dementia. These mice develop a neurological illness with prominent ataxia at 65 or 240 days of age, depending on whether the transgene array is, respectively, homozygous or hemizygous. Starting from birth, mutant PrP is converted into a protease-resistant and detergent-insoluble form that resembles the scrapie isoform of PrP, and this form accumulates dramatically in many brain regions throughout the lifetime of the mice. As PrP accumulates, there is massive apoptosis of granule cells in the cerebellum. Our analysis provides important insights into the molecular pathogenesis of inherited prion disorders in humans.
Figures
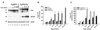

References
-
- Young K, Piccardo P, Dlouhy S, Bugiani O, Tagliavini F, Ghetti B. In: Prions: Molecular and Cellular Biology. Harris D A, editor. Wymondham: Horizon Scientific Press; 1999. pp. 139–175.
-
- Gambetti P, Petersen R B, Parchi P, Chen S G, Capellari S, Goldfarb L, Gabizon R, Montagna P, Lugaresi E, Piccardo P, Ghetti B. In: Prion Biology and Diseases. Prusiner S B, editor. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1999. pp. 509–583.
-
- Chiesa R, Piccardo P, Ghetti B, Harris D A. Neuron. 1998;21:1339–1351. - PubMed
-
- Owen F, Poulter M, Collinge J, Leach M, Lofthouse R, Crow T J, Harding A E. Mol Brain Res. 1992;13:155–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

