Ectopic deposition of lignin in the pith of stems of two Arabidopsis mutants
- PMID: 10806225
- PMCID: PMC58982
- DOI: 10.1104/pp.123.1.59
Ectopic deposition of lignin in the pith of stems of two Arabidopsis mutants
Abstract
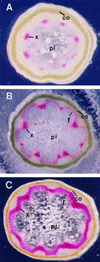
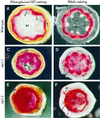
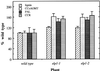
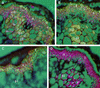
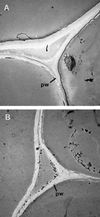
The biosynthesis of lignin in vascular plants is regulated both developmentally and environmentally. In the inflorescence stems of Arabidopsis, lignin is mainly deposited in the walls of xylem cells and interfascicular fiber cells during normal plant growth and development. The mechanisms controlling the spatial deposition of lignin remain unknown. By screening ethyl methanesulfonate-mutagenized populations of Arabidopsis, we have isolated two allelic elp1 (ectopic deposition of lignin in pith) mutants with altered lignin deposition patterns. In elp1 stems, lignin was ectopically deposited in the walls of pith parenchyma cells in addition to its normal deposition in the walls of xylem and fiber cells. Lignin appeared to be deposited in patches of parenchyma cells in the pith of both young and mature elp1 stems. The ectopic deposition of lignin in the pith of elp1 stems was accompanied by an increase in the activities of enzymes in the lignin biosynthetic pathway and with the ectopic expression of caffeoyl coenzyme A O-methyltransferase in pith cells. These results indicate that the ELP1 locus is involved in the repression of the lignin biosynthetic pathway in the pith. Isolation of the elp1 mutants provides a novel means with which to study the molecular mechanisms underlying the spatial control of lignification.
Figures

Similar articles
-
The genetic control of lignin deposition during plant growth and development.New Phytol. 2004 Oct;164(1):17-30. doi: 10.1111/j.1469-8137.2004.01143.x. New Phytol. 2004. PMID: 33873487 Review.
-
Mutation of a chitinase-like gene causes ectopic deposition of lignin, aberrant cell shapes, and overproduction of ethylene.Plant Cell. 2002 Jan;14(1):165-79. doi: 10.1105/tpc.010278. Plant Cell. 2002. PMID: 11826306 Free PMC article.
-
Defining the Diverse Cell Populations Contributing to Lignification in Arabidopsis Stems.Plant Physiol. 2017 Jun;174(2):1028-1036. doi: 10.1104/pp.17.00434. Epub 2017 Apr 17. Plant Physiol. 2017. PMID: 28416705 Free PMC article.
-
Evidence for a role of AtCAD 1 in lignification of elongating stems of Arabidopsis thaliana.Planta. 2006 Dec;225(1):23-39. doi: 10.1007/s00425-006-0326-9. Epub 2006 Jul 11. Planta. 2006. PMID: 16832689
-
A review of xylan and lignin biosynthesis: foundation for studying Arabidopsis irregular xylem mutants with pleiotropic phenotypes.Crit Rev Biochem Mol Biol. 2014 May-Jun;49(3):212-41. doi: 10.3109/10409238.2014.889651. Epub 2014 Feb 24. Crit Rev Biochem Mol Biol. 2014. PMID: 24564339 Review.
Cited by
-
Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene.Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15270-5. doi: 10.1073/pnas.0707294104. Epub 2007 Sep 19. Proc Natl Acad Sci U S A. 2007. PMID: 17881564 Free PMC article.
-
Isolation of rice dwarf mutants with ectopic deposition of phenolic components including lignin in parenchyma cell walls of internodes.Plant Cell Rep. 2011 Dec;30(12):2195-205. doi: 10.1007/s00299-011-1125-8. Epub 2011 Jul 29. Plant Cell Rep. 2011. PMID: 21800099
-
Characterization of the ABC Transporter G Subfamily in Pomegranate and Function Analysis of PgrABCG14.Int J Mol Sci. 2022 Oct 1;23(19):11661. doi: 10.3390/ijms231911661. Int J Mol Sci. 2022. PMID: 36232964 Free PMC article.
-
The genetic control of lignin deposition during plant growth and development.New Phytol. 2004 Oct;164(1):17-30. doi: 10.1111/j.1469-8137.2004.01143.x. New Phytol. 2004. PMID: 33873487 Review.
-
Genetic structure and molecular mechanism underlying the stalk lodging traits in maize (Zea mays L.).Comput Struct Biotechnol J. 2022 Dec 21;21:485-494. doi: 10.1016/j.csbj.2022.12.037. eCollection 2023. Comput Struct Biotechnol J. 2022. PMID: 36618981 Free PMC article.
References
-
- Akin DE, Hanna WW, Snook ME, Himmelsbach DS, Barton FE, Windham WR. Normal-12 and brown midrib-12 sorghum: II. Chemical variations and digestibility. Agron J. 1986;78:832–837.
-
- Bender J, Fink GR. Epigenetic control of an endogenous gene family is revealed by a novel blue fluorescent mutant of Arabidopsis. Cell. 1995;83:725–734. - PubMed
-
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

