Expression of water channel proteins in Mesembryanthemum crystallinum
- PMID: 10806230
- PMCID: PMC58987
- DOI: 10.1104/pp.123.1.111
Expression of water channel proteins in Mesembryanthemum crystallinum
Abstract
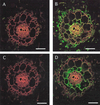
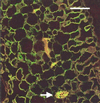
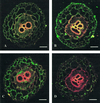
We have characterized transcripts for nine major intrinsic proteins (MIPs), some of which function as water channels (aquaporins), from the ice plant Mesembryanthemum crystallinum. To determine the cellular distribution and expression of these MIPs, oligopeptide-based antibodies were generated against MIP-A, MIP-B, MIP-C, or MIP-F, which, according to sequence and functional characteristics, are located in the plasma membrane (PM) and tonoplast, respectively. MIPs were most abundant in cells involved in bulk water flow and solute flux. The tonoplast MIP-F was found in all cells, while signature cell types identified different PM-MIPs: MIP-A predominantly in phloem-associated cells, MIP-B in xylem parenchyma, and MIP-C in the epidermis and endodermis of immature roots. Membrane protein analysis confirmed MIP-F as tonoplast located. MIP-A and MIP-B were found in tonoplast fractions and also in fractions distinct from either the tonoplast or PM. MIP-C was most abundant but not exclusive to PM fractions, where it is expected based on its sequence signature. We suggest that within the cell, MIPs are mobile, which is similar to aquaporins cycling through animal endosomes. MIP cycling and the differential regulation of these proteins observed under conditions of salt stress may be fundamental for the control of tissue water flux.
Figures






References
-
- Abrami L, Capurro C, Ibarra C, Parisi M, Buhler JM, Ripoche P. Distribution of mRNA encoding the FA-CHIP water channel in amphibian tissues: effects of salt adaptation. J Membr Biol. 1995;143:199–205. - PubMed
-
- Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H. Growth and development of Mesembryanthemum crystallinum (Aizoaceae) New Phytol. 1998;138:171–190. - PubMed
-
- Agre P, Bonhivers M, Borgnia MJ. The aquaporins, blueprints for cellular plumbing systems. J Biol Chem. 1998;273:14659–14662. - PubMed
-
- Barkla BJ, Vera-Estrella R, Kirch HH, Pantoja O, Bohnert HJ. Aquaporin localization: how valid are the TIP and PIP labels? Trends Plant Sci. 1999;4:86–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

