The BtpA protein stabilizes the reaction center proteins of photosystem I in the cyanobacterium Synechocystis sp. PCC 6803 at low temperature
- PMID: 10806238
- PMCID: PMC58995
- DOI: 10.1104/pp.123.1.215
The BtpA protein stabilizes the reaction center proteins of photosystem I in the cyanobacterium Synechocystis sp. PCC 6803 at low temperature
Abstract
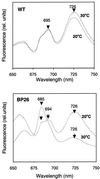
Specific inhibition of photosystem I (PSI) was observed under low-temperature conditions in the cyanobacterium Synechocystis sp. strain PCC 6803. Growth at 20 degrees C caused inhibition of PSI activity and increased degradation of the PSI reaction center proteins PsaA and PsaB, while no significant changes were found in the level and activity of photosystem II (PSII). BtpA, a recently identified extrinsic thylakoid membrane protein, was found to be a necessary regulatory factor for stabilization of the PsaA and PsaB proteins under such low-temperature conditions. At normal growth temperature (30 degrees C), the BtpA protein was present in the cell, and its genetic deletion caused an increase in the degradation of the PSI reaction center proteins. However, growth of Synechocystis cells at 20 degrees C or shifting of cultures grown at 30 degrees C to 20 degrees C led to a rapid accumulation of the BtpA protein, presumably to stabilize the PSI complex, by lowering the rates of degradation of the PsaA and PsaB proteins. A btpA deletion mutant strain could not grow photoautotrophically at low temperature, and exhibited rapid degradation of the PSI complex after transfer of the cells from normal to low temperature.
Figures





Similar articles
-
Subcellular localization of the BtpA protein in the cyanobacterium Synechocystis sp. PCC 6803.Eur J Biochem. 1999 Apr;261(1):311-6. doi: 10.1046/j.1432-1327.1999.00281.x. Eur J Biochem. 1999. PMID: 10103064
-
Molecular identification of a novel protein that regulates biogenesis of photosystem I, a membrane protein complex.J Biol Chem. 1997 Mar 7;272(10):6382-7. doi: 10.1074/jbc.272.10.6382. J Biol Chem. 1997. PMID: 9045660
-
Absence of PsaC subunit allows assembly of photosystem I core but prevents the binding of PsaD and PsaE in Synechocystis sp. PCC6803.Plant Mol Biol. 1995 Oct;29(2):331-42. doi: 10.1007/BF00043656. Plant Mol Biol. 1995. PMID: 7579183
-
Genetic inactivation of the psaB gene in Synechocystis sp. PCC 6803 disrupts assembly of photosystem I.Plant Mol Biol. 1993 Jan;21(1):177-80. doi: 10.1007/BF00039628. Plant Mol Biol. 1993. PMID: 8425045
-
Bidirectional electron transfer in photosystem I: electron transfer on the PsaA side is not essential for phototrophic growth in Chlamydomonas.Biochim Biophys Acta. 2003 Sep 30;1606(1-3):43-55. doi: 10.1016/s0005-2728(03)00083-5. Biochim Biophys Acta. 2003. PMID: 14507426 Review.
Cited by
-
Loss of Albino3 leads to the specific depletion of the light-harvesting system.Plant Cell. 2002 Sep;14(9):2303-14. doi: 10.1105/tpc.003442. Plant Cell. 2002. PMID: 12215522 Free PMC article.
-
One of two alb3 proteins is essential for the assembly of the photosystems and for cell survival in Chlamydomonas.Plant Cell. 2006 Jun;18(6):1454-66. doi: 10.1105/tpc.105.038695. Epub 2006 May 5. Plant Cell. 2006. PMID: 16679460 Free PMC article.
-
Hypothetical chloroplast reading frame 51 encodes a photosystem I assembly factor in cyanobacteria.Plant Cell. 2024 May 1;36(5):1844-1867. doi: 10.1093/plcell/koad330. Plant Cell. 2024. PMID: 38146915 Free PMC article.
-
A Novel Redoxin in the Thylakoid Membrane Regulates the Titer of Photosystem I.J Biol Chem. 2016 Sep 2;291(36):18689-99. doi: 10.1074/jbc.M116.721175. Epub 2016 Jul 5. J Biol Chem. 2016. PMID: 27382055 Free PMC article.
-
Structure, function, and assembly of PSI in thylakoid membranes of vascular plants.Plant Cell. 2024 Oct 3;36(10):4080-4108. doi: 10.1093/plcell/koae169. Plant Cell. 2024. PMID: 38848316 Free PMC article. Review.
References
-
- Allen MM. Simple conditions for growth of unicellular blue-green algae on plates. J Phycol. 1968;4:1–4. - PubMed
-
- Aro E-M, Virgin I, Andersson B. Photoinhibition of photosystem II: inactivation, protein damage, and turnover. Biochim Biophys Acta. 1993;1143:113–134. - PubMed
-
- Bartsevich VV, Pakrasi HB. Molecular identification of a novel protein that regulates biogenesis of photosystem I, a membrane protein complex. J Biol Chem. 1997;272:6382–6387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

