Purification and characterization of a novel pumpkin short-chain acyl-coenzyme A oxidase with structural similarity to acyl-coenzyme A dehydrogenases
- PMID: 10806249
- PMCID: PMC59006
- DOI: 10.1104/pp.123.1.327
Purification and characterization of a novel pumpkin short-chain acyl-coenzyme A oxidase with structural similarity to acyl-coenzyme A dehydrogenases
Abstract
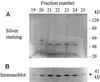
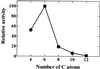
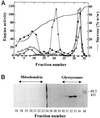
A novel pumpkin (Cucurbita pepo) short-chain acyl-coenzyme A (CoA) oxidase (ACOX) was purified to homogeneity by hydrophobic-interaction, hydroxyapatite, affinity, and anion-exchange chromatography. The purified enzyme is a tetrameric protein, consisting of apparently identical 47-kD subunits. The protein structure of this oxidase differs from other plant and mammalian ACOXs, but is similar to the protein structure of mammalian mitochondrial acyl-CoA dehydrogenase (ACDH) and the recently identified plant mitochondrial ACDH. Subcellular organelle separation by sucrose density gradient centrifugation revealed that the enzyme is localized in glyoxysomes, whereas no immunoreactive bands of similar molecular weight were detected in mitochondrial fractions. The enzyme selectively catalyzes the oxidation of CoA esters of fatty acids with 4 to 10 carbon atoms, and exhibits the highest activity on C-6 fatty acids. Apparently, the enzyme has no activity on CoA esters of branched-chain or dicarboxylic fatty acids. The enzyme is slightly inhibited by high concentrations of substrate and it is not inhibited by Triton X-100 at concentrations up to 0.5% (v/v). The characteristics of this novel ACOX enzyme are discussed in relation to other ACOXs and ACDHs.
Figures




Similar articles
-
The CoA esters of 2-methyl-branched chain fatty acids and of the bile acid intermediates di- and trihydroxycoprostanic acids are oxidized by one single peroxisomal branched chain acyl-CoA oxidase in human liver and kidney.J Biol Chem. 1993 May 15;268(14):10335-44. J Biol Chem. 1993. PMID: 8387517
-
Plant acyl-CoA oxidase. Purification, characterization, and monomeric apoprotein.J Biol Chem. 1986 Jun 25;261(18):8570-5. J Biol Chem. 1986. PMID: 3087978
-
Three-dimensional structure of the flavoenzyme acyl-CoA oxidase-II from rat liver, the peroxisomal counterpart of mitochondrial acyl-CoA dehydrogenase.J Biochem. 2002 Mar;131(3):365-74. doi: 10.1093/oxfordjournals.jbchem.a003111. J Biochem. 2002. PMID: 11872165
-
Acyl-CoA dehydrogenases and acyl-CoA oxidases. Structural basis for mechanistic similarities and differences.Eur J Biochem. 2004 Feb;271(3):483-93. doi: 10.1046/j.1432-1033.2003.03948.x. Eur J Biochem. 2004. PMID: 14728675 Review.
-
[Peroxisomal beta-oxidation].Verh K Acad Geneeskd Belg. 1993;55(1):45-78. Verh K Acad Geneeskd Belg. 1993. PMID: 8480447 Review. Dutch.
Cited by
-
Functional Characterization of GhACX3 Gene Reveals Its Significant Role in Enhancing Drought and Salt Stress Tolerance in Cotton.Front Plant Sci. 2021 Aug 10;12:658755. doi: 10.3389/fpls.2021.658755. eCollection 2021. Front Plant Sci. 2021. PMID: 34447398 Free PMC article.
-
Storage reserve mobilisation and seedling establishment in Arabidopsis.Arabidopsis Book. 2006;4:e0100. doi: 10.1199/tab.0100. Epub 2006 Oct 4. Arabidopsis Book. 2006. PMID: 22303229 Free PMC article. No abstract available.
-
Gene isolation and characterization of two acyl CoA oxidases from soybean with broad substrate specificities and enhanced expression in the growing seedling axis.Plant Mol Biol. 2001 Nov;47(4):519-31. doi: 10.1023/a:1011825114301. Plant Mol Biol. 2001. PMID: 11669577
-
Acyl-CoA dehydrogenases: Dynamic history of protein family evolution.J Mol Evol. 2009 Aug;69(2):176-93. doi: 10.1007/s00239-009-9263-0. Epub 2009 Jul 29. J Mol Evol. 2009. PMID: 19639238 Free PMC article.
-
ATP allosterically regulates an acyl-CoA oxidase.Nat Commun. 2025 Aug 8;16(1):7318. doi: 10.1038/s41467-025-61905-9. Nat Commun. 2025. PMID: 40781076 Free PMC article.
References
-
- Däschner K, Thalheim C, Guha C, Brennicke A, Binder S. In plants a putative isovaleryl-CoA dehydrogenase is located in mitochondria. Plant Mol Biol. 1999;39:1275–1282. - PubMed
-
- De Bellis L, Giuntini P, Hayashi H, Hayashi M, Nishimura M. Purification and characterization of pumpkin long-chain acyl-CoA oxidase. Physiol Plant. 1999;106:170–176.
-
- Dommes V, Kunau WH. A convenient assay for acyl-CoA-dehydrogenase. Anal Biochem. 1976;71:571–578. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

