Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial
- PMID: 10807619
- PMCID: PMC27372
- DOI: 10.1136/bmj.320.7245.1297
Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial
Abstract
Objectives: To determine the effect of long term inhaled corticosteroids on lung function, exacerbations, and health status in patients with moderate to severe chronic obstructive pulmonary disease.
Design: Double blind, placebo controlled study.
Setting: Eighteen UK hospitals.
Participants: 751 men and women aged between 40 and 75 years with mean forced expiratory volume in one second (FEV(1)) 50% of predicted normal.
Interventions: Inhaled fluticasone propionate 500 microgram twice daily from a metered dose inhaler or identical placebo.
Main outcome measures: Efficacy measures: rate of decline in FEV(1) after the bronchodilator and in health status, frequency of exacerbations, respiratory withdrawals. Safety measures: morning serum cortisol concentration, incidence of adverse events.
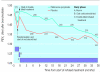
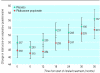
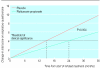
Results: There was no significant difference in the annual rate of decline in FEV(1 )(P=0.16). Mean FEV(1) after bronchodilator remained significantly higher throughout the study with fluticasone propionate compared with placebo (P<0.001). Median exacerbation rate was reduced by 25% from 1.32 a year on placebo to 0.99 a year on with fluticasone propionate (P=0.026). Health status deteriorated by 3.2 units a year on placebo and 2.0 units a year on fluticasone propionate (P=0.0043). Withdrawals because of respiratory disease not related to malignancy were higher in the placebo group (25% v 19%, P=0.034).
Conclusions: Fluticasone propionate 500 microgram twice daily did not affect the rate of decline in FEV(1) but did produce a small increase in FEV(1). Patients on fluticasone propionate had fewer exacerbations and a slower decline in health status. These improvements in clinical outcomes support the use of this treatment in patients with moderate to severe chronic obstructive pulmonary disease.
Figures
Comment in
-
The ISOLDE trial. Side effects with inhaled steroids should not be forgotten.BMJ. 2000 Nov 25;321(7272):1349. BMJ. 2000. PMID: 11186442 Free PMC article. No abstract available.
-
The ISOLDE trial. Side effects are source of concern.BMJ. 2000 Nov 25;321(7272):1349. BMJ. 2000. PMID: 11186443 No abstract available.
-
The ISOLDE trial. Pharmaceutical companies should admit high cost of treatment.BMJ. 2000 Nov 25;321(7272):1349-50. BMJ. 2000. PMID: 11186444 No abstract available.
References
-
- Thom TJ. International comparisons in COPD mortality. Am Rev Respir Dis. 1989;140:27–34. - PubMed
-
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. - PubMed
-
- Murray CJL, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet. 1997;349:1498–1504. - PubMed
-
- Jones PW, Quirk FH, Baveystock CM. The St George's respiratory questionnaire. Respir Med. 1991;85(suppl B):25–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
