Size and quality of randomised controlled trials in head injury: review of published studies
- PMID: 10807622
- PMCID: PMC27374
- DOI: 10.1136/bmj.320.7245.1308
Size and quality of randomised controlled trials in head injury: review of published studies
Abstract
Objective: To assess whether trials in head injury are large enough to avoid moderate random errors and designed to avoid moderate biases.
Design: All randomised controlled trials on the treatment and rehabilitation of patients with head injury published before December 1998 were surveyed. Trials were identified from electronic databases, by hand searching journals and conference proceedings, and by contacting researchers. Data were extracted on the number of participants, quality of concealment of allocation, use of blinding, loss to follow up, and types of participants, interventions, and outcome measures.
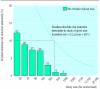
Results: 279 reports were identified, containing information on 208 separate trials. The average number of participants per trial was 82, with no evidence of increasing size over time. The total number of randomised participants in the 203 trials in which size was reported was 16 613. No trials were large enough to detect reliably a 5% absolute reduction in the risk of death or disability, and only 4% were large enough to detect an absolute reduction of 10%. Concealment of allocation was adequate in 22 and inadequate or unclear in 25 of the 47 (23%) in which it was reported. Of 126 trials assessing disability, 111 reported the number of patients followed up, and average loss to follow up was 19%. Of trials measuring disability, 26 (21%) reported that outcome assessors were blinded.
Conclusions: Randomised trials in head injury are too small and poorly designed to detect or refute reliably moderate but clinically important benefits or hazards of treatment. Limited funding for injury research and unfamiliarity with issues of consent may have been important obstacles.
Figures
Comment in
-
Quality of randomised controlled trials in head injury. Trials in head injury are more complex than review suggests.BMJ. 2000 Nov 11;321(7270):1223. BMJ. 2000. PMID: 11073525 Free PMC article. No abstract available.
-
Quality of randomised controlled trials in head injury. If in doubt, declare competing interests.BMJ. 2000 Nov 11;321(7270):1223-4. BMJ. 2000. PMID: 11073526 No abstract available.
-
Quality of randomised controlled trials in head injury. More trials are needed.BMJ. 2000 Sep 16;321(7262):704. BMJ. 2000. PMID: 11202935 No abstract available.
-
Quality of randomised controlled trials in head injury. Statistical power can be increased.BMJ. 2000 Sep 16;321(7262):704. BMJ. 2000. PMID: 11202936 Free PMC article. No abstract available.
References
-
- Murray CJL, Lopez AD. Global health statistics: a compendium of incidence, prevalence and mortality estimates for over 200 conditions. Boston: Harvard University Press; 1996.
-
- World Bank. Global road safety partnership. Washington, DC: World Bank; 1999.
-
- Peto R, Collins R, Gray R. Large-scale randomised evidence: large simple trials and overviews of trials. J Clin Epidemiol. 1995;48:23–40. - PubMed
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. - PubMed
-
- Teasdale G. The treatment of head trauma: implications for the future. J Neurotrauma. 1991;8(suppl 1):53–60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical