Suppression of arterial intimal hyperplasia by cilostamide, a cyclic nucleotide phosphodiesterase 3 inhibitor, in a rat balloon double-injury model
- PMID: 10807659
- PMCID: PMC1572059
- DOI: 10.1038/sj.bjp.0703287
Suppression of arterial intimal hyperplasia by cilostamide, a cyclic nucleotide phosphodiesterase 3 inhibitor, in a rat balloon double-injury model
Abstract
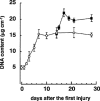
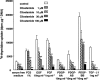
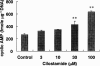
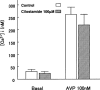
The effects of cilostamide, a cyclic nucleotide phosphodiesterase 3 (PDE3) selective inhibitor, on vascular intimal hyperplasia were evaluated using a single-balloon injury model and a double-injury model in which the rat common carotid artery was subjected to a second injury at a site injured 14 days previously. In the double-injury model, the second balloon injury caused more severe intimal hyperplasia (intima/media (IM) ratio, 1.88+/-0.10) than in the single-injury model (1.09+/-0.08). Histopathological study revealed that vascular smooth muscle cells (VSMC) were the predominant cell-type in the affected neointimal area. Oral administration of cilostamide for 2 weeks after the second injury suppressed intimal hyperplasia in the double-injury model (30 mg kg(-1) bid, 83% inhibition in terms of the IM ratio, P<0.05; 100 mg kg(-1) bid, 69% inhibition, P<0.05). Similar effects were also observed in the single-injury model with oral administration of cilostamide for 2 weeks (100 mg kg(-1) bid, 36% inhibition, P<0.01). Cilostamide inhibited DNA synthesis of cultured VSMC stimulated by foetal calf serum or different kinds of growth factors, but did not affect their migration stimulated by platelet-derived growth factor (PDGF)-BB. Cilostamide significantly increased the cyclic AMP concentration of VSMC dose-dependently. These results indicate that cilostamide suppresses intimal hyperplasia both in the single- and double-injury models of rat, presumably by inhibiting proliferation rather than migration of VSMC. It is suggested that PDE3 inhibitors might find application in preventing intimal hyperplasia following angioplasty such as percutaneous transluminal coronary angioplasty (PTCA) or stent.
Figures




Similar articles
-
Tranilast suppresses intimal hyperplasia in the balloon injury model and cuff treatment model in rabbits.Jpn J Pharmacol. 1996 Apr;70(4):321-7. doi: 10.1254/jjp.70.321. Jpn J Pharmacol. 1996. PMID: 8774760
-
Effects of a single local administration of cilostazol on neointimal formation in balloon-injured rat carotid artery.Atherosclerosis. 1999 Jan;142(1):41-6. doi: 10.1016/s0021-9150(98)00147-6. Atherosclerosis. 1999. PMID: 9920504
-
N-oleoylethanolamide suppresses intimal hyperplasia after balloon injury in rats through AMPK/PPARα pathway.Biochem Biophys Res Commun. 2018 Feb 5;496(2):415-421. doi: 10.1016/j.bbrc.2018.01.015. Epub 2018 Jan 4. Biochem Biophys Res Commun. 2018. PMID: 29305859
-
The long and short of vascular smooth muscle phosphodiesterase-4 as a putative therapeutic target.Mol Pharmacol. 2005 Sep;68(3):563-7. doi: 10.1124/mol.105.015719. Epub 2005 Jun 15. Mol Pharmacol. 2005. PMID: 15958394 Review.
-
Postoperative Intimal Hyperplasia: Review from My Research.Int J Angiol. 2024 Feb 5;33(3):135-138. doi: 10.1055/s-0044-1779300. eCollection 2024 Sep. Int J Angiol. 2024. PMID: 39131804 Free PMC article. Review.
Cited by
-
Phosphodiesterase 3A (PDE3A) deletion suppresses proliferation of cultured murine vascular smooth muscle cells (VSMCs) via inhibition of mitogen-activated protein kinase (MAPK) signaling and alterations in critical cell cycle regulatory proteins.J Biol Chem. 2011 Jul 22;286(29):26238-49. doi: 10.1074/jbc.M110.214155. Epub 2011 Jun 1. J Biol Chem. 2011. PMID: 21632535 Free PMC article.
-
Role of PDE3A in regulation of cell cycle progression in mouse vascular smooth muscle cells and oocytes: implications in cardiovascular diseases and infertility.Curr Opin Pharmacol. 2011 Dec;11(6):725-9. doi: 10.1016/j.coph.2011.10.006. Epub 2011 Nov 1. Curr Opin Pharmacol. 2011. PMID: 22051884 Free PMC article. Review.
-
Lentiviral-Mediated shRNA Silencing of PDE4D Gene Inhibits Platelet-Derived Growth Factor-Induced Proliferation and Migration of Rat Aortic Smooth Muscle Cells.Stroke Res Treat. 2011;2011:534257. doi: 10.4061/2011/534257. Epub 2011 Jun 9. Stroke Res Treat. 2011. PMID: 21776361 Free PMC article.
-
Roles of A-Kinase Anchoring Proteins and Phosphodiesterases in the Cardiovascular System.J Cardiovasc Dev Dis. 2018 Feb 20;5(1):14. doi: 10.3390/jcdd5010014. J Cardiovasc Dev Dis. 2018. PMID: 29461511 Free PMC article. Review.
-
Phosphodiesterase in heart and vessels: from physiology to diseases.Physiol Rev. 2024 Apr 1;104(2):765-834. doi: 10.1152/physrev.00015.2023. Epub 2023 Nov 16. Physiol Rev. 2024. PMID: 37971403 Free PMC article. Review.
References
-
- BAPTISTA J., UMANS V.A., DI MARIO C., ESCANED J., DE FEYTER P., SERRUYS P.W. Mechanism of luminal enlargement and quantification of vessel wall trauma following balloon coronary angioplasty and directional atherectomy. Eur. Heart. J. 1995;16:1603–1612. - PubMed
-
- BEAVO J.A., CONTI M., HEASLIP R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994;46:399–405. - PubMed
-
- BONAN R., PAIEMENT P., LEUNG T.K. Swine model of coronary restenosis: effect of a second injury. Cathet. Cardiovasc. Diagn. 1996;38:44–49. - PubMed
-
- CAPRON L., JARNET J., HEUDES D., MONROSE D.J., BRUNEVAL P. Repeated balloon injury of rat aorta: A model of neointima with attenuated inhibition by heparin. Arterioscler. Thromb. Vasc. Biol. 1997;17:1649–1656. - PubMed
-
- CLOWES A.W., SCHWARTZ S.M. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ. Res. 1985;56:139–145. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

