Non-NF-kappaB elements are required for full induction of the rat type II nitric oxide synthase in vascular smooth muscle cells
- PMID: 10807663
- PMCID: PMC1572057
- DOI: 10.1038/sj.bjp.0703284
Non-NF-kappaB elements are required for full induction of the rat type II nitric oxide synthase in vascular smooth muscle cells
Abstract
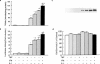
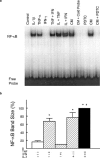
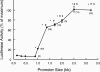
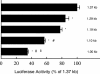
We have investigated the role of the NF-kappaB binding sites and other promoter elements beyond NF-kappaB in iNOS induction in rat vascular smooth muscle cells (SMC). Rat aortic SMC transfected with iNOS promoter constructs with either mutation or deletion of the downstream NF-kappaB site exhibited about 50% reduction in promoter activity in response to a cytokine mixture, whereas either mutation or deletion of the upstream NF-kappaB site reduced promoter activity by 90%, suggesting that the latter site is the most important, and that co-existence of two NF-kappaB sites is necessary for iNOS induction. Nuclear NF-kappaB activity was robustly induced by TNF-alpha. However, TNF-alpha alone did not induce iNOS promoter activity, protein expression, or nitrite production, indicating that NF-kappaB activation alone is not sufficient for iNOS induction. The construct up to -890 bp, containing the downstream NF-kappaB site, exhibited little response to cytokines. The construct up to -1.0 kb, containing the two NF-kappaB sites exhibited only 22% of full promoter activity. The regions -1001 to -1368 bp and -2 to -2.5 kb contributed an additional 43 and 22% promoter activity, respectively. Internal deletion or reversal of the orientation of -1001 to -1368 bp in the full promoter resulted in 40% reduction in promoter activity. These data suggest that the co-existence of two NF-kappaB sites is essential for core promoter activity, but that full induction of the rat SMC iNOS gene requires other elements located between -1.0 to -1.37 and -2.0 to -2.5 kb of the promoter.
Figures



Similar articles
-
Molecular cloning and analysis of the rat inducible nitric oxide synthase gene promoter in aortic smooth muscle cells.Biochem Pharmacol. 1998 Jun 1;55(11):1873-80. doi: 10.1016/s0006-2952(98)00078-1. Biochem Pharmacol. 1998. PMID: 9714306
-
Role of nuclear factor-kappaB activation in cytokine- and sphingomyelinase-stimulated inducible nitric oxide synthase gene expression in vascular smooth muscle cells.Endocrinology. 1998 Nov;139(11):4506-12. doi: 10.1210/endo.139.11.6309. Endocrinology. 1998. PMID: 9794459
-
A reverse nuclear factor-kappaB element in the rat type II nitric oxide synthase promoter mediates the induction by interleukin-1beta and interferon-gamma in rat aortic smooth muscle cells.Gen Pharmacol. 2000 Jan;34(1):9-16. doi: 10.1016/s0306-3623(99)00047-6. Gen Pharmacol. 2000. PMID: 10793263
-
Cytokine-activated p42/p44 MAP kinase is involved in inducible nitric oxide synthase gene expression independent from NF-kappaB activation in vascular smooth muscle cells.Hypertens Res. 2000 Nov;23(6):659-67. doi: 10.1291/hypres.23.659. Hypertens Res. 2000. PMID: 11131279
-
Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene.Shock. 2000 Jun;13(6):413-24. doi: 10.1097/00024382-200006000-00001. Shock. 2000. PMID: 10847627 Review.
Cited by
-
Identification of conserved domains in the promoter regions of nitric oxide synthase 2: implications for the species-specific transcription and evolutionary differences.BMC Genomics. 2007 Aug 8;8:271. doi: 10.1186/1471-2164-8-271. BMC Genomics. 2007. PMID: 17686182 Free PMC article.
References
-
- ATCHISON M.L. Enhancers: mechanisms of action and cell specificity. Annu. Rev. Cell Biol. 1988;4:127–153. - PubMed
-
- BECK K.F., STERZEL R.B. Cloning and sequencing of the proximal promoter of the rat iNOS gene: activation of NFkappaB is not sufficient for transcription of the iNOS gene in rat mesangial cells. FEBS Lett. 1996;394:263–267. - PubMed
-
- CARMICHAEL J., DEGRAFF W.G., GAZDAR A.F., MINNA J.D., MITCHELL J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. - PubMed
-
- DE VERA M.E., SHAPIRO R.A., NUSSLER A.K., MUDGETT J.S., SIMMONS R.L., MORRIS S.M., BILLIAR T.R., JR, GELLER D.A. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1054–1059. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

