Induction of skeletal muscle contracture and calcium release from isolated sarcoplasmic reticulum vesicles by sanguinarine
- PMID: 10807666
- PMCID: PMC1572056
- DOI: 10.1038/sj.bjp.0703279
Induction of skeletal muscle contracture and calcium release from isolated sarcoplasmic reticulum vesicles by sanguinarine
Abstract
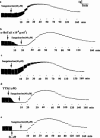
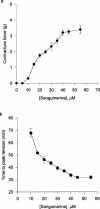
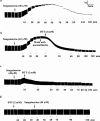
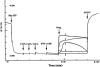
The benzophenanthrine alkaloid, sanguinarine, was studied for its effects on isolated mouse phrenic-nerve diaphragm preparations. Sanguinarine induced direct, dose-dependent effects on muscle contractility. Sanguinarine-induced contracture was partially inhibited when the extracellular Ca(2+) was removed or when the diaphragm was pretreated with nifedipine. Depletion of sarcoplasmic reticulum (SR) internal calcium stores completely blocked the contracture. Sanguinarine induced Ca(2+) release from the actively loaded SR vesicles was blocked by ruthenium red and dithiothreitol (DTT), consistent with the ryanodine receptor (RyR) as the site of sanguinarine action. Sanguinarine altered [(3)H]-ryanodine binding to the RyR of isolated SR vesicles, potentiating [(3)H]-ryanodine binding at lower concentrations and inhibiting binding at higher concentrations. All of these effects were reversed by DTT, suggesting that sanguinarine-induced Ca(2+) release from SR occurs through oxidation of critical SH groups of the RyR SR calcium release channel.
Figures
Similar articles
-
Emodin-induced muscle contraction of mouse diaphragm and the involvement of Ca2+ influx and Ca2+ release from sarcoplasmic reticulum.Br J Pharmacol. 1998 Mar;123(5):815-20. doi: 10.1038/sj.bjp.0701677. Br J Pharmacol. 1998. PMID: 9535008 Free PMC article.
-
Induction of calcium release from sarcoplasmic reticulum of skeletal muscle by xanthone and norathyriol.Br J Pharmacol. 1996 Aug;118(7):1736-42. doi: 10.1111/j.1476-5381.1996.tb15599.x. Br J Pharmacol. 1996. PMID: 8842439 Free PMC article.
-
Effects of cartap on isolated mouse phrenic nerve diaphragm and its related mechanism.Toxicol Sci. 2000 Jun;55(2):453-9. doi: 10.1093/toxsci/55.2.453. Toxicol Sci. 2000. PMID: 10828278
-
Effects of boldine on mouse diaphragm and sarcoplasmic reticulum vesicles isolated from skeletal muscle.Planta Med. 1998 Feb;64(1):18-21. doi: 10.1055/s-2006-957358. Planta Med. 1998. PMID: 9491763
-
Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):889-96. doi: 10.1111/j.1440-1681.2007.04708.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645636 Review.
Cited by
-
Use of Evidence-Based Herbal Medicines for Patients with Functional Gastrointestinal Disorders: A Conceptional Framework for Risk-Benefit Assessment and Regulatory Approaches.Dig Dis. 2020;38(4):269-279. doi: 10.1159/000504570. Epub 2019 Nov 26. Dig Dis. 2020. PMID: 31770769 Free PMC article. Review.
-
The effect of chelerythrine on depolarization-induced force responses in skinned fast skeletal muscle fibres of the rat.Br J Pharmacol. 2003 Feb;138(3):417-26. doi: 10.1038/sj.bjp.0705035. Br J Pharmacol. 2003. PMID: 12569066 Free PMC article.
-
Effects of concentric and eccentric contractions on phosphorylation of MAPK(erk1/2) and MAPK(p38) in isolated rat skeletal muscle.J Physiol. 2001 Aug 15;535(Pt 1):155-64. doi: 10.1111/j.1469-7793.2001.00155.x. J Physiol. 2001. PMID: 11507166 Free PMC article.
-
Antitumor effects of the benzophenanthridine alkaloid sanguinarine: Evidence and perspectives.World J Gastrointest Oncol. 2016 Jan 15;8(1):30-9. doi: 10.4251/wjgo.v8.i1.30. World J Gastrointest Oncol. 2016. PMID: 26798435 Free PMC article. Review.
References
-
- ABRAMSON J.J., BUCK E., SALAMA G., CASIDA J.E., PESSAH I.N. Mechanism of anthraquinone-induced calcium release from skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1988;263:18750–18758. - PubMed
-
- ABRAMSON J.J., SALAMA G. Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J. Bioenerg Biomembr. 1989;21:283–294. - PubMed
-
- AGARWAL S., REYNOLDS M.A., POU S., PETERSON D.E., CHARON J.A., SUZUKI J.B. The effect of sanguinarine on human peripheral blood neutrophil viability and functions. Oral Microbiol. Immunol. 1991;6:51–61. - PubMed
-
- AGHDASI B., ZHANG J.Z., WU Y., REID M.B., HAMILTON S.L. Multiple classes of sulfhydryls modulate the skeletal muscle Ca2+ release channel. J. Biol. Chem. 1997;272:3739–3748. - PubMed
-
- BECCI P.J., SCHWARTZ H., BARNES H.H., SOUTHARD G.L. Short-term toxicity studies of sanguinarine and of two alkaloid extracts of Sanguinaria canadesis L. J. Toxicol. Environ. Health. 1987;20:199–208. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

