Effects of a quaternary bupivacaine derivative on delayed rectifier K(+) currents
- PMID: 10807678
- PMCID: PMC1572085
- DOI: 10.1038/sj.bjp.0703334
Effects of a quaternary bupivacaine derivative on delayed rectifier K(+) currents
Abstract
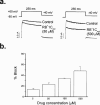
Block of hKv1.5 channels by R-bupivacaine has been attributed to the interaction of the charged form of the drug with an intracellular receptor. However, bupivacaine is present as a mixture of neutral and charged forms both extra- and intracellularly. We have studied the effects produced by the R(+) enantiomer of a quaternary bupivacaine derivative, N-methyl-bupivacaine, (RB(+)1C) on hKv1.5 channels stably expressed in Ltk(-) cells using the whole-cell configuration of the patch-clamp technique. When applied from the intracellular side of the membrane, RB(+)1C induced a time- and voltage-dependent block similar to that induced by R-bupivacaine. External application of 50 microM RB(+)1C reduced the current at +60 mV by 24+/-2% (n=10), but this block displayed neither time- nor voltage-dependence. External RB(+)1C partially relieved block induced by R-bupivacaine (61+/-2% vs 56+/-3%, n=4, P<0.05), but it did not relieve block induced by internal RB(+)1C. In addition, it did not induce use-dependent block, but when applied in combination with internal RB(+)1C a use-dependent block that increased with pulse duration was observed. These results indicate that RB(+)1C induces different effects on hKv1.5 channels when applied from the intra or the extracellular side of the membrane, suggesting that the actions of bupivacaine are the resulting of those induced on the external and the internal side of hKv1.5 channels.
Figures








References
-
- BAUKROWITZ T., YELLEN G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
-
- BAUKROWITZ T., YELLEN G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science. 1996a;271:653–656. - PubMed
-
- CASTLE N.A. Bupivacaine inhibits the transient outward K+ current but not the inward rectifier in rat ventricular myocytes. J. Pharmacol. Exp. Ther. 1990;255:1038–1046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

