Reversal of the vasoconstrictor step of hyperacute xenogeneic rejection of the liver by endothelin antagonists
- PMID: 10807679
- PMCID: PMC1572063
- DOI: 10.1038/sj.bjp.0703295
Reversal of the vasoconstrictor step of hyperacute xenogeneic rejection of the liver by endothelin antagonists
Abstract
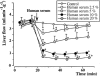
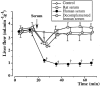
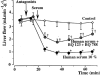
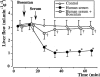
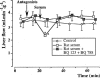
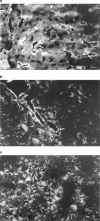
The role of endothelin in the initial vasoconstrictor step of hyperacute xenogeneic rejection was investigated. Isolated rat livers were perfused in recirculation. Perfusion with human sera provided an ex vivo model of hyperacute rejection in a discordant combination. Perfusion of 10% xenogeneic serum induced a marked (70%) and sustained reduction of the liver flow and induced the release of endothelin into the perfusion medium. In contrast, perfusion of 10% allogeneic serum or of 10% decomplemented human serum induced a weak (25%) and transient reduction of the liver flow and induced the release of minimal amounts of endothelin. The simultaneous administration of BQ 123 and BQ 788, the respective antagonists of ET(A) and ET(B) endothelin receptors, or that of bosentan, a mixed ET(A)/ET(B) antagonist, antagonized the vasoconstrictor effect of 10% xenogeneic human serum, as well as that of 10(-9) M endothelin-1. The vasoconstrictor effects of xenogeneic serum on liver circulation are, at least partly, mediated through the release of endothelin by the graft.
Figures



Similar articles
-
Hyperacute rejection in the xenogenic transplanted rat liver is triggered by the complement system only in the presence of leukocytes and free radical species.Xenotransplantation. 2013 May-Jun;20(3):177-87. doi: 10.1111/xen.12035. Epub 2013 May 9. Xenotransplantation. 2013. PMID: 23656281
-
Endothelin receptor A blockade ameliorates hypothermic ischemia-reperfusion-related microhemodynamic disturbances during liver transplantation in the rat.J Surg Res. 2002 Feb;102(2):63-70. doi: 10.1006/jsre.2001.6246. J Surg Res. 2002. PMID: 11796000
-
Early endothelin production during hyperacute xenogeneic rejection of the liver.Transplant Proc. 1994 Jun;26(3):1078. Transplant Proc. 1994. PMID: 8029836 No abstract available.
-
Endothelins and the lung.Int J Biochem Cell Biol. 2000 Jan;32(1):41-62. doi: 10.1016/s1357-2725(99)00115-6. Int J Biochem Cell Biol. 2000. PMID: 10661893 Review.
-
Endothelin receptor antagonists: a new therapeutic option for improving the outcome after solid organ transplantation?Curr Vasc Pharmacol. 2003 Oct;1(3):281-99. doi: 10.2174/1570161033476655. Curr Vasc Pharmacol. 2003. PMID: 15320475 Review.
References
-
- ARAI H., HORI S., ARAMORI I., OHKUBO H., NAKANISHI S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. - PubMed
-
- AUCHINCLOSS H. Xenografting: a review. Transplantation. 1988;46:1–20. - PubMed
-
- BALLET F., CHRÉTIEN Y., REY C., POUPON R. Norepinephrine: a potential modulator of the hepatic transport of taurocholate. A study in the isolated perfused rat liver. J. Pharmacol. Exp. Ther. 1987;240:303–307. - PubMed
-
- BRAUER R.W., LEONG G.F., PESSOTTI R.L. Vasomotor activity in the isolated perfused rat liver. Am. J. Physiol. 1953;174:304–312. - PubMed
-
- GANDHI C.R., STEPHENSON K., OLSON M.S. Endothelin, a potent peptide agonist in the liver. J. Biol. Chem. 1990;265:17432–17435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

