The phosphatidylinositol 4-kinase inhibitor phenylarsine oxide blocks evoked neurotransmitter release by reducing calcium entry through N-type calcium channels
- PMID: 10807681
- PMCID: PMC1572064
- DOI: 10.1038/sj.bjp.0703299
The phosphatidylinositol 4-kinase inhibitor phenylarsine oxide blocks evoked neurotransmitter release by reducing calcium entry through N-type calcium channels
Abstract
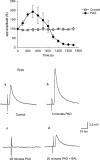
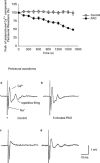
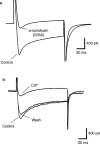
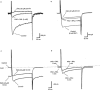
The effects of the phosphatidylinositol 4-kinase inhibitor, phenylarsine oxide (PAO), on acetylcholine (ACh) release and on prejunctional Ca(2+) currents were studied at the frog neuromuscular junction using electrophysiological recording techniques. Application of PAO (30 microM) increased both spontaneous ACh release reflected as miniature end-plate potential (mepp) frequencies and evoked ACh release reflected as end-plate potential (epp) amplitudes with a similar time course. Following the initial increase in epp amplitudes produced by PAO, epps slowly declined and were eventually abolished after approximately 20 min. However, mepp frequencies remained elevated over this time period. PAO (30 microM) also inhibited the perineural voltage change associated with Ca(2+) currents through N-type Ca(2+) channels (prejunctional Ca(2+) currents) at motor nerve endings. Addition of British anti-lewisite (BAL, 1 mM), an inactivator of PAO, partially reversed both the inhibition of epps and the inhibition of the prejunctional Ca(2+) current. The effects of PAO on N-type Ca(2+) channels were investigated more directly using the whole cell patch clamp technique on acutely dissociated sympathetic neurons. Application of PAO (30 - 40 microM) to these neurons decreased the voltage-activated calcium currents through N-type Ca(2+) channels, an effect that was partially reversible by BAL. In combination, these results suggest that inhibition of neurotransmitter release by PAO occurs as a consequence of the inhibition of Ca(2+) entry via N-type calcium channels. The relationship between the effects of PAO on N-type Ca(2+) channels in motor nerve endings and in neuronal soma is discussed.
Figures
Similar articles
-
Antagonism of calcium currents and neurotransmitter release by barium ions at frog motor nerve endings.Br J Pharmacol. 2000 Jan;129(2):360-6. doi: 10.1038/sj.bjp.0703036. Br J Pharmacol. 2000. PMID: 10694243 Free PMC article.
-
Opposing effects of phorbol esters on transmitter release and calcium currents at frog motor nerve endings.J Physiol. 1997 May 15;501 ( Pt 1)(Pt 1):41-8. doi: 10.1111/j.1469-7793.1997.041bo.x. J Physiol. 1997. PMID: 9174992 Free PMC article.
-
On the simultaneous electrophysiological measurements of neurotransmitter release and perineural calcium currents from frog motor nerve endings.J Neurosci Methods. 1995 Apr;57(2):151-9. doi: 10.1016/0165-0270(94)00133-2. J Neurosci Methods. 1995. PMID: 7609578
-
Influence of heavy metals on synaptic transmission: a review.Neurotoxicology. 1983 Winter;4(4):69-83. Neurotoxicology. 1983. PMID: 6322059 Review.
-
Calcium current in motor nerve endings of the lizard.Ann N Y Acad Sci. 1991;635:58-69. doi: 10.1111/j.1749-6632.1991.tb36481.x. Ann N Y Acad Sci. 1991. PMID: 1683760 Review. No abstract available.
Cited by
-
Intranasal administration as a route for drug delivery to the brain: evidence for a unique pathway for albumin.J Pharmacol Exp Ther. 2014 Oct;351(1):54-60. doi: 10.1124/jpet.114.216705. Epub 2014 Jul 15. J Pharmacol Exp Ther. 2014. PMID: 25027317 Free PMC article.
-
The sympathetic nervous system regulates skeletal muscle motor innervation and acetylcholine receptor stability.Acta Physiol (Oxf). 2019 Mar;225(3):e13195. doi: 10.1111/apha.13195. Epub 2018 Oct 22. Acta Physiol (Oxf). 2019. PMID: 30269419 Free PMC article.
-
Phosphatidylinositol-4,5-Biphosphate (PI(4,5)P2) Is Required for Rapid Endocytosis in Chromaffin Cells.Biomed Res Int. 2020 Sep 9;2020:9692503. doi: 10.1155/2020/9692503. eCollection 2020. Biomed Res Int. 2020. PMID: 32964048 Free PMC article.
-
Pi4KIIα Regulates Unconditioned Stimulus-Retrieval-Induced Fear Memory Reconsolidation through Endosomal Trafficking of AMPA Receptors.iScience. 2020 Mar 27;23(3):100895. doi: 10.1016/j.isci.2020.100895. Epub 2020 Feb 8. iScience. 2020. PMID: 32088394 Free PMC article.
References
-
- BARAJAS-LOPEZ C., PERES A.L., ESPINOSA-LUNA R. Cellular mechanisms underlying adenosine actions on cholinergic transmission in enteric neurons. Am. J. Physiol. (Cell Physiol 40) 1996;271:C263–C275. - PubMed
-
- BENNETT M.K. Calcium and the regulation of neurotransmitter secretion. Curr. Opin. Neurobiol. 1998;7:316–322. - PubMed
-
- DE CAMILLI P., EMR S.D., MCPHERSON P.S., NOVICK P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

