A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana
- PMID: 10809670
- PMCID: PMC316574
A subtilisin-like serine protease involved in the regulation of stomatal density and distribution in Arabidopsis thaliana
Abstract
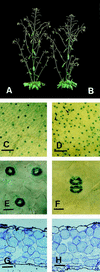
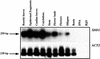
Stomata are specialized cellular structures in the epidermis of aerial plant organs that control gas exchange (H(2)O release and CO(2) uptake) between leaves and the atmosphere by modulating the aperture of a pore flanked by two guard cells. Stomata are nonrandomly distributed, and their density is controlled by endogenous and environmental factors. To gain insight into the molecular mechanisms regulating stomatal distribution, Arabidopsis thaliana mutants with altered stomatal characteristics were isolated and examined. The sdd1-1 mutant exhibits a two- to fourfold increase of stomatal density and formation of clustered stomata (i.e., stomata that are not separated by intervening pavement cells), whereas the internal leaf architecture is not altered. The SDD1 gene was identified by map-based cloning. It encodes a subtilisin-like serine protease related to prokaryotic and eukaryotic proteins. We propose that SDD1 acts as a processing protease involved in the mediation of a signal that controls the development of cell lineages that lead to guard cell formation.
Figures



References
-
- Abak K, Yanmaz R. Investigation on the stomatal density in certain pepper lines and their F1 hybrids. Capsicum Newslett. 1985;4:22.
-
- An Y-Q, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB. Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant j. 1996;10:107–121. - PubMed
-
- Barr PJ. Mammalian subtilisins: The long-sought dibasic processing endoproteases. Cell. 1991;66:1–3. - PubMed
-
- Bechthold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. CR Acad Sci. 1993;316:1194–1199.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
