SPC4/PACE4 regulates a TGFbeta signaling network during axis formation
- PMID: 10809672
- PMCID: PMC316583
SPC4/PACE4 regulates a TGFbeta signaling network during axis formation
Abstract
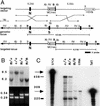
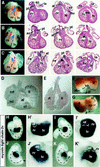
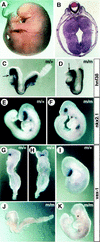
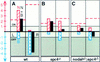
In vertebrates, specification of anteroposterior (A/P) and left-right (L/R) axes depends on TGFbeta-related signals, including Nodal, Lefty, and BMPs. Endoproteolytic maturation of these proteins is probably mediated by the proprotein convertase SPC1/Furin. In addition, precursor processing may be regulated by related activities such as SPC4 (also known as PACE4). Here, we show that a proportion of embryos lacking SPC4 develop situs ambiguus combined with left pulmonary isomerism or complex craniofacial malformations including cyclopia, or both. Gene expression analysis during early somite stages indicates that spc4 is genetically upstream of nodal, pitx2, lefty1, and lefty2 and perhaps maintains the balance between Nodal and BMP signaling in the lateral plate that is critical for L/R axis formation. Furthermore, genetic interactions between nodal and spc4, together with our analysis of chimeric embryos, strongly suggest that during A/P axis formation, SPC4 acts primarily in the foregut. These findings establish an important role for SPC4 in patterning the early mouse embryo.
Figures



Similar articles
-
Extraembryonic proteases regulate Nodal signalling during gastrulation.Nat Cell Biol. 2002 Dec;4(12):981-5. doi: 10.1038/ncb890. Nat Cell Biol. 2002. PMID: 12447384
-
Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases.J Cell Biol. 1999 Jan 11;144(1):139-49. doi: 10.1083/jcb.144.1.139. J Cell Biol. 1999. PMID: 9885250 Free PMC article.
-
Role of asymmetric signals in left-right patterning in the mouse.Am J Med Genet. 2001 Jul 15;101(4):324-7. Am J Med Genet. 2001. PMID: 11471154
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
TGFβ signaling in establishing left-right asymmetry.Semin Cell Dev Biol. 2014 Aug;32:80-4. doi: 10.1016/j.semcdb.2014.03.029. Epub 2014 Apr 2. Semin Cell Dev Biol. 2014. PMID: 24704359 Review.
Cited by
-
Loss-of-function mutations in growth differentiation factor-1 (GDF1) are associated with congenital heart defects in humans.Am J Hum Genet. 2007 Nov;81(5):987-94. doi: 10.1086/522890. Epub 2007 Sep 28. Am J Hum Genet. 2007. PMID: 17924340 Free PMC article.
-
An activin/furin regulatory loop modulates the processing and secretion of inhibin alpha- and betaB-subunit dimers in pituitary gonadotrope cells.J Biol Chem. 2008 Nov 28;283(48):33059-68. doi: 10.1074/jbc.M804190200. Epub 2008 Sep 30. J Biol Chem. 2008. PMID: 18826955 Free PMC article.
-
BMP/SMAD1 signaling sets a threshold for the left/right pathway in lateral plate mesoderm and limits availability of SMAD4.Genes Dev. 2008 Nov 1;22(21):3037-49. doi: 10.1101/gad.1682108. Genes Dev. 2008. PMID: 18981480 Free PMC article.
-
A role for PACE4 in osteoarthritis pain: evidence from human genetic association and null mutant phenotype.Ann Rheum Dis. 2012 Jun;71(6):1042-8. doi: 10.1136/annrheumdis-2011-200300. Epub 2012 Mar 22. Ann Rheum Dis. 2012. PMID: 22440827 Free PMC article.
-
Beyond TGFβ: roles of other TGFβ superfamily members in cancer.Nat Rev Cancer. 2013 May;13(5):328-41. doi: 10.1038/nrc3500. Nat Rev Cancer. 2013. PMID: 23612460 Free PMC article. Review.
References
-
- Beddington RSP, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. - PubMed
-
- Benjannet S, Savaria D, Laslop A, Munzer JS, Chretien M, Marcinkiewicz M, Seidah NG. Alpha1-antitrypsin Portland inhibits processing of precursors mediated by proprotein convertases primarily within the constitutive secretory pathway. J Biol Chem. 1997;272:26210–26218. - PubMed
-
- Bisgrove BW, Essner JJ, Yost HJ. Regulation of midline development by antagonism of lefty and nodal signaling. Development. 1999;126:3253–3262. - PubMed
-
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. - PubMed
-
- Chang H, Zwijsen A, Vogel H, Huylebroeck D, Matzuk MM. Smad5 is essential for left-right asymmetry in mice. Dev Biol. 2000;219:71–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
