Physical mapping of bchG, orf427, and orf177 in the photosynthesis gene cluster of Rhodobacter sphaeroides: functional assignment of the bacteriochlorophyll synthetase gene
- PMID: 10809697
- PMCID: PMC94504
- DOI: 10.1128/JB.182.11.3175-3182.2000
Physical mapping of bchG, orf427, and orf177 in the photosynthesis gene cluster of Rhodobacter sphaeroides: functional assignment of the bacteriochlorophyll synthetase gene
Abstract
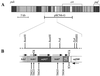
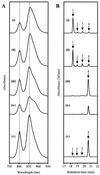
The purple photosynthetic bacterium Rhodobacter sphaeroides has within its genome a cluster of photosynthesis-related genes approximately 41 kb in length. In an attempt to identify genes involved in the terminal esterification stage of bacteriochlorophyll biosynthesis, a previously uncharacterized 5-kb region of this cluster was sequenced. Four open reading frames (ORFs) were identified, and each was analyzed by transposon mutagenesis. The product of one of these ORFs, bchG, shows close homologies with (bacterio)chlorophyll synthetases, and mutants in this gene were found to accumulate bacteriopheophorbide, the metal-free derivative of the bacteriochlorophyll precursor bacteriochlorophyllide, suggesting that bchG is responsible for the esterification of bacteriochlorophyllide with an alcohol moiety. This assignment of function to bchG was verified by the performance of assays demonstrating the ability of BchG protein, heterologously synthesized in Escherichia coli, to esterify bacteriochlorophyllide with geranylgeranyl pyrophosphate in vitro, thereby generating bacteriochlorophyll. This step is pivotal to the assembly of a functional photosystem in R. sphaeroides, a model organism for the study of structure-function relationships in photosynthesis. A second gene, orf177, is a member of a large family of isopentenyl diphosphate isomerases, while sequence homologies suggest that a third gene, orf427, may encode an assembly factor for photosynthetic complexes. The function of the remaining ORF, bchP, is the subject of a separate paper (H. Addlesee and C. N. Hunter, J. Bacteriol. 181:7248-7255, 1999). An operonal arrangement of the genes is proposed.
Figures



Similar articles
-
Physical mapping and functional assignment of the geranylgeranyl-bacteriochlorophyll reductase gene, bchP, of Rhodobacter sphaeroides.J Bacteriol. 1999 Dec;181(23):7248-55. doi: 10.1128/JB.181.23.7248-7255.1999. J Bacteriol. 1999. PMID: 10572128 Free PMC article.
-
An uncultivated crenarchaeota contains functional bacteriochlorophyll a synthase.ISME J. 2009 Jan;3(1):106-16. doi: 10.1038/ismej.2008.85. Epub 2008 Oct 2. ISME J. 2009. PMID: 18830277
-
The Photoheterotrophic Growth of Bacteriochlorophyll Synthase-Deficient Mutant of Rhodobacter sphaeroides Is Restored by I44F Mutant Chlorophyll Synthase of Synechocystis sp. PCC 6803.J Microbiol Biotechnol. 2016 May 28;26(5):959-66. doi: 10.4014/jmb.1601.01019. J Microbiol Biotechnol. 2016. PMID: 26869605
-
Sequencing, chromosomal inactivation, and functional expression in Escherichia coli of ppsR, a gene which represses carotenoid and bacteriochlorophyll synthesis in Rhodobacter sphaeroides.J Bacteriol. 1994 May;176(10):2869-76. doi: 10.1128/jb.176.10.2869-2876.1994. J Bacteriol. 1994. PMID: 8188588 Free PMC article.
-
Genetic evidence for the role of isocytochrome c2 in photosynthetic growth of Rhodobacter sphaeroides Spd mutants.J Bacteriol. 1993 Jan;175(2):358-66. doi: 10.1128/jb.175.2.358-366.1993. J Bacteriol. 1993. PMID: 8380401 Free PMC article.
Cited by
-
A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis.Plant Physiol. 2007 Sep;145(1):29-40. doi: 10.1104/pp.107.100321. Epub 2007 May 25. Plant Physiol. 2007. PMID: 17535821 Free PMC article.
-
Rhodospirillum rubrum possesses a variant of the bchP gene, encoding geranylgeranyl-bacteriopheophytin reductase.J Bacteriol. 2002 Mar;184(6):1578-86. doi: 10.1128/JB.184.6.1578-1586.2002. J Bacteriol. 2002. PMID: 11872709 Free PMC article.
-
Identification of the bchP gene, encoding geranylgeranyl reductase in Chlorobaculum tepidum.J Bacteriol. 2008 Jan;190(2):747-9. doi: 10.1128/JB.01430-07. Epub 2007 Nov 9. J Bacteriol. 2008. PMID: 17993528 Free PMC article.
-
The terminal enzymes of (bacterio)chlorophyll biosynthesis.R Soc Open Sci. 2022 May 4;9(5):211903. doi: 10.1098/rsos.211903. eCollection 2022 May. R Soc Open Sci. 2022. PMID: 35573041 Free PMC article. Review.
-
Lack of Phosphatidylglycerol Inhibits Chlorophyll Biosynthesis at Multiple Sites and Limits Chlorophyllide Reutilization in Synechocystis sp. Strain PCC 6803.Plant Physiol. 2015 Oct;169(2):1307-17. doi: 10.1104/pp.15.01150. Epub 2015 Aug 12. Plant Physiol. 2015. PMID: 26269547 Free PMC article.
References
-
- Addlesee H A, Gibson L C D, Jensen P E, Hunter C N. Cloning, sequencing and functional assignment of the chlorophyll biosynthesis gene, chlP, of Synechocystis sp. PCC 6803. FEBS Lett. 1996;389:126–130. - PubMed
-
- Alberti M, Burke D H, Hearst J E. Structure and sequence of the photosynthesis gene cluster. In: Blankenship R E, Madigan M T, Bauer C E, editors. Anoxygenic photosynthetic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 1083–1106.
-
- Ashby M N, Edwards P A. Elucidation of the deficiency in two yeast coenzyme Q mutants: characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J Biol Chem. 1990;265:13157–13164. - PubMed
-
- Bauer C E, Buggy J J, Yang Z, Marrs B L. The superoperonal organization of genes for pigment biosynthesis and reaction center proteins is a conserved feature in Rhodobacter capsulatus: analysis of overlapping bchB and puhA transcripts. Mol Gen Genet. 1991;228:433–444. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

