Another unusual type of citric acid cycle enzyme in Helicobacter pylori: the malate:quinone oxidoreductase
- PMID: 10809701
- PMCID: PMC94508
- DOI: 10.1128/JB.182.11.3204-3209.2000
Another unusual type of citric acid cycle enzyme in Helicobacter pylori: the malate:quinone oxidoreductase
Abstract
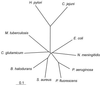
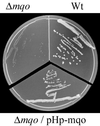
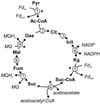
The only enzyme of the citric acid cycle for which no open reading frame (ORF) was found in the Helicobacter pylori genome is the NAD-dependent malate dehydrogenase. Here, it is shown that in this organism the oxidation of malate to oxaloacetate is catalyzed by a malate:quinone oxidoreductase (MQO). This flavin adenine dinucleotide-dependent membrane-associated enzyme donates electrons to quinones of the electron transfer chain. Similar to succinate dehydrogenase, it is part of both the electron transfer chain and the citric acid cycle. MQO activity was demonstrated in isolated membranes of H. pylori. The enzyme is encoded by the ORF HP0086, which is shown by the fact that expression of the HP0086 sequence from a plasmid induces high MQO activity in mqo deletion mutants of Escherichia coli or Corynebacterium glutamicum. Furthermore, this plasmid was able to complement the phenotype of the C. glutamicum mqo deletion mutant. Interestingly, the protein predicted to be encoded by this ORF is only distantly related to known or postulated MQO sequences from other bacteria. The presence of an MQO shown here and the previously demonstrated presence of a 2-ketoglutarate:ferredoxin oxidoreductase and a succinyl-coenzyme A (CoA):acetoacetyl-CoA transferase indicate that H. pylori possesses a complete citric acid cycle, but one which deviates from the standard textbook example in three steps.
Figures
References
-
- Abe S, Takayama K, Kinoshita S. Taxonomical studies on glutamic acid producing organisms. J Gen Appl Microbiol. 1967;13:279–301.
-
- Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, de Jonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. - PubMed
-
- Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and mu. J Mol Biol. 1976;104:541–555. - PubMed
-
- Chang H T, Marcelli S W, Davison A A, Chalk P A, Poole R K, Miles R J. Kinetics of substrate oxidation by whole cells and cell membranes of Helicobacter pylori. FEMS Microbiol Lett. 1995;129:33–38. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

