Mutational analysis of the sbo-alb locus of Bacillus subtilis: identification of genes required for subtilosin production and immunity
- PMID: 10809709
- PMCID: PMC94516
- DOI: 10.1128/JB.182.11.3266-3273.2000
Mutational analysis of the sbo-alb locus of Bacillus subtilis: identification of genes required for subtilosin production and immunity
Abstract
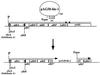
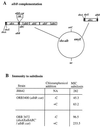
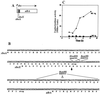
The Bacillus subtilis 168 derivative JH642 produces a bacteriocin, subtilosin, which possesses activity against Listeria monocytogenes. Inspection of the amino acid sequence of the presubtilosin polypeptide encoded by the gene sboA and sequence data from analysis of mature subtilosin indicate that the precursor subtilosin peptide undergoes several unique and unusual chemical modifications during its maturation process. The genes of the sbo-alb operon are believed to function in the synthesis and maturation of subtilosin. Nonpolar mutations introduced into each of the alb genes resulted in loss or reduction of subtilosin production. sboA, albA, and albF mutants showed no antilisterial activity, indicating that the products of these genes are critical for the production of active subtilosin. Mutations in albB, -C, and -D resulted in reduction of antilisterial activity and decreased immunity to subtilosin, particularly under anaerobic conditions. A new gene, sboX, encoding another bacteriocin-like product was discovered residing in a sequence overlapping the coding region of sboA. Construction of an sboX-lacZ translational fusion and analysis of its expression indicate that sboX is induced in stationary phase of anaerobic cultures of JH642. An in-frame deletion of the sboX coding sequence did not affect the antilisterial activity or production of or immunity to subtilosin. The results of this investigation show that the sbo-alb genes are required for the mechanisms of subtilosin synthesis and immunity.
Figures

Similar articles
-
Genes of the sbo-alb locus of Bacillus subtilis are required for production of the antilisterial bacteriocin subtilosin.J Bacteriol. 1999 Dec;181(23):7346-55. doi: 10.1128/JB.181.23.7346-7355.1999. J Bacteriol. 1999. PMID: 10572140 Free PMC article.
-
Dual control of sbo-alb operon expression by the Spo0 and ResDE systems of signal transduction under anaerobic conditions in Bacillus subtilis.J Bacteriol. 2000 Jun;182(11):3274-7. doi: 10.1128/JB.182.11.3274-3277.2000. J Bacteriol. 2000. PMID: 10809710 Free PMC article.
-
Isolation of a variant of subtilosin A with hemolytic activity.J Bacteriol. 2009 Sep;191(18):5690-6. doi: 10.1128/JB.00541-09. Epub 2009 Jul 24. J Bacteriol. 2009. PMID: 19633086 Free PMC article.
-
Subtilosin production by two Bacillus subtilis subspecies and variance of the sbo-alb cluster.Appl Environ Microbiol. 2004 Apr;70(4):2349-53. doi: 10.1128/AEM.70.4.2349-2353.2004. Appl Environ Microbiol. 2004. PMID: 15066831 Free PMC article.
-
Genetics of subtilin and nisin biosyntheses: biosynthesis of lantibiotics.Antonie Van Leeuwenhoek. 1996 Feb;69(2):109-17. doi: 10.1007/BF00399416. Antonie Van Leeuwenhoek. 1996. PMID: 8775971 Review.
Cited by
-
Mechanism of Action of Ribosomally Synthesized and Post-Translationally Modified Peptides.Chem Rev. 2022 Sep 28;122(18):14722-14814. doi: 10.1021/acs.chemrev.2c00210. Epub 2022 Sep 1. Chem Rev. 2022. PMID: 36049139 Free PMC article. Review.
-
Genome Mining Reveals a Sactipeptide Biosynthetic Cluster in Staphylococcus pseudintermedius.Vet Sci. 2025 Jul 2;12(7):635. doi: 10.3390/vetsci12070635. Vet Sci. 2025. PMID: 40711295 Free PMC article.
-
Radical S-adenosylmethionine enzymes.Chem Rev. 2014 Apr 23;114(8):4229-317. doi: 10.1021/cr4004709. Epub 2014 Jan 29. Chem Rev. 2014. PMID: 24476342 Free PMC article. Review. No abstract available.
-
Revealing nature's synthetic potential through the study of ribosomal natural product biosynthesis.ACS Chem Biol. 2013 Mar 15;8(3):473-87. doi: 10.1021/cb3005325. Epub 2013 Jan 8. ACS Chem Biol. 2013. PMID: 23286465 Free PMC article. Review.
-
Occurrence, function, and biosynthesis of mycofactocin.Appl Microbiol Biotechnol. 2019 Apr;103(7):2903-2912. doi: 10.1007/s00253-019-09684-4. Epub 2019 Feb 18. Appl Microbiol Biotechnol. 2019. PMID: 30778644 Free PMC article. Review.
References
-
- Babasaki K, Takao T, Shimonishi Y, Kurahashi K. Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: isolation, structural analysis, and biogenesis. J Biochem. 1985;98:583–603. - PubMed
-
- Beuchat L R. Traditional fermented foods. In: Doyle M P, Beuchat L R, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C.: American Society for Microbiology; 1997. pp. 629–648.
-
- Dubnau D, Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. formation and properties of the donor-recipient complex. J Mol Biol. 1971;56:209–221. - PubMed
-
- Goodwin P M, Anthony C. The biochemistry, physiology and genetics of PQQ and PQQ-containing enzymes. Adv Microb Physiol. 1998;40:1–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

