Lysosomal cathepsin B plays an important role in antigen processing, while cathepsin D is involved in degradation of the invariant chain inovalbumin-immunized mice
- PMID: 10809954
- PMCID: PMC2326990
- DOI: 10.1046/j.1365-2567.2000.00000.x
Lysosomal cathepsin B plays an important role in antigen processing, while cathepsin D is involved in degradation of the invariant chain inovalbumin-immunized mice
Abstract
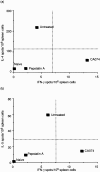
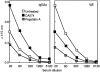
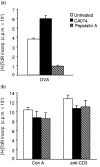
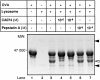
We previously reported that CA074, a specific inhibitor of cathepsin B, modulates specific immune responses from the T helper 2 (Th2) type to Th1 type in BALB/c mice infected with Leishmania major. In the present study, we found that a similar type of immune deviation was also induced in mice immunized with ovalbumin (OVA). However, treatment of mice with pepstatin A, a specific cathepsin D inhibitor, suppressed the OVA-specific proliferation of lymphocytes and blocked the development of both Th1 and Th2 cellular responses. These inhibitors did not appear to have any direct influence in vitro on functions of naive lymphocytes. OVA antigen (47 000 MW) was digested mainly into 40 000 MW protein in vitro by lysosomal proteases from naive BALB/c mice, and its digestion was markedly inhibited by the addition of CA074, but not by addition of pepstatin A, during incubation. However, pepstatin A strongly suppressed the degradation of the major histocompatibility complex class II-associated invariant chain (Ii) molecule in vivo and in vitro. Thus, cathepsin B appears to process antigens directed to preferential activation of Th2 cells, while cathepsin D may be responsible for the degradation of Ii, the processing of which is essential in initiating the antigen-specific activation of Th1 and Th2 CD4+ T cells. These lysosomal proteases may have different functions in regulating immune responses.
Figures


References
-
- Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821. - PubMed
-
- Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287. - PubMed
-
- Puri J, Factorovich Y. Selective inhibition of antigen presentation to cloned T cells by protease inhibitors. J Immunol. 1988;141:3313. - PubMed
-
- Takahashi H, Cease KB, Berzofsky JA. Identification of proteases that process distinct epitopes on the same protein. J Immunol. 1989;142:2221. - PubMed
-
- Rodriguez GM, Diment S. Destructive proteolysis by cysteine proteases in antigen presentation of ovalbumin. Eur J Immunol. 1995;25:1823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

