CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes
- PMID: 10809957
- PMCID: PMC2326993
- DOI: 10.1046/j.1365-2567.2000.00001.x
CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes
Abstract
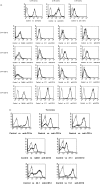
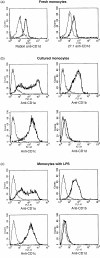
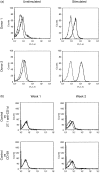
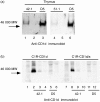
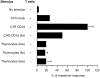
Human T cells expressing CD161 and an invariant T-cell receptor (TCR) alpha-chain (Valpha24invt T cells) specifically recognize CD1d and appear to have immunoregulatory functions. However, the physiological target cells for this T-cell population, and whether alterations in CD1d expression contribute to the regulation of Valpha24invt T-cell responses, remain to be determined. A series of antibodies were generated to assess CD1d expression, structure and regulation on human lymphoid and myeloid cells. CD1d was expressed at high levels by human cortical thymocytes and immunoprecipitation analyses showed it to be a 48 000-MW glycosylated protein. However, after solubilization, the majority of the thymocyte CD1d protein, but not CD1d expressed by transfected cells, lost reactivity with monoclonal antibodies (mAbs) against native CD1d, indicating that it was alternatively processed. Moreover, thymocytes were not recognized by CD1d-reactive Valpha24invt T-cell clones. Medullary thymocytes and resting peripheral blood T cells were CD1d-, but low-level CD1d expression was induced on activated T cells. CD1d was expressed by B cells in peripheral blood and lymph node mantle zones, but germinal centres were CD1d-. Resting monocytes were CD1d+ but, in contrast to CD1a, b and c, their surface expression of CD1d was not up-regulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) activation. These results demonstrate constitutive CD1d expression by human professional antigen-presenting cells and that post-translational processing of CD1d may contribute to regulation of the activity of CD1d-specific T cells.
Figures


Similar articles
-
CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant V alpha 24 J alpha Q T cell receptor alpha chains.J Exp Med. 1998 Sep 7;188(5):867-76. doi: 10.1084/jem.188.5.867. J Exp Med. 1998. PMID: 9730888 Free PMC article.
-
Expression of a gp33/27,000 MW activation inducer molecule (AIM) on human lymphoid tissues. Induction of cell proliferation on thymocytes and B lymphocytes by anti-AIM antibodies.Immunology. 1989 Sep;68(1):72-9. Immunology. 1989. PMID: 2807372 Free PMC article.
-
Antigen-specific regulation of CD1 expression in humans.J Clin Immunol. 2000 May;20(3):203-11. doi: 10.1023/a:1006689514066. J Clin Immunol. 2000. PMID: 10941828
-
Structure and function of the CD1 family of MHC-like cell surface proteins.Immunol Rev. 1995 Oct;147:5-29. doi: 10.1111/j.1600-065x.1995.tb00085.x. Immunol Rev. 1995. PMID: 8847079 Review.
-
Antigen specificity of semi-invariant CD1d-restricted T cell receptors: the best of both worlds?Immunol Cell Biol. 2004 Jun;82(3):285-94. doi: 10.1111/j.0818-9641.2004.01257.x. Immunol Cell Biol. 2004. PMID: 15186260 Review.
Cited by
-
'Off-the-shelf' allogeneic CAR T cells: development and challenges.Nat Rev Drug Discov. 2020 Mar;19(3):185-199. doi: 10.1038/s41573-019-0051-2. Epub 2020 Jan 3. Nat Rev Drug Discov. 2020. PMID: 31900462 Review.
-
Potent NKT cell ligands overcome SARS-CoV-2 immune evasion to mitigate viral pathogenesis in mouse models.PLoS Pathog. 2023 Mar 24;19(3):e1011240. doi: 10.1371/journal.ppat.1011240. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36961850 Free PMC article.
-
Human CD4+ iNKT cell adoptive immunotherapy induces anti-tumour responses against CD1d-negative EBV-driven B lymphoma.Immunology. 2024 Aug;172(4):627-640. doi: 10.1111/imm.13799. Epub 2024 May 13. Immunology. 2024. PMID: 38736328 Free PMC article.
-
T cells and T cell tumors efficiently generate antigen-specific cytotoxic T cell immunity when modified with an NKT ligand.Oncoimmunology. 2012 Mar 1;1(2):141-151. doi: 10.4161/onci.1.2.18479. Oncoimmunology. 2012. PMID: 22720235 Free PMC article.
-
Current Perspectives on the Use of off the Shelf CAR-T/NK Cells for the Treatment of Cancer.Cancers (Basel). 2021 Apr 16;13(8):1926. doi: 10.3390/cancers13081926. Cancers (Basel). 2021. PMID: 33923528 Free PMC article. Review.
References
-
- Aruffo A, Seed B. Expression of cDNA clones encoding the thymocyte antigens CD1a, b, c demonstrates a hierarchy of exclusion in fibroblasts. J Immunol. 1989;143:1723. - PubMed
-
- McMichael AJ, Pilch JR, Galfre G, Mason DY, Fabre JW, Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979;9:205. - PubMed
-
- Kahn-perles B, Wietzerbin J, Caillol DH, Lemonnier F. Delineation of three subsets of class I human T antigens (HTA) on Molt-4 cells: serologic and regulatory relationship to HLA class I antigens. J Immunol. 1985;134:1759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

