Expression of cytokine genes in Marek's disease virus-infected chickens and chicken embryo fibroblast cultures
- PMID: 10809961
- PMCID: PMC2326989
- DOI: 10.1046/j.1365-2567.2000.00008.x
Expression of cytokine genes in Marek's disease virus-infected chickens and chicken embryo fibroblast cultures
Abstract
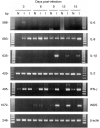
The role of cytokines in the pathogenesis and immunity of Marek's disease (MD), a herpesvirus-induced T-cell lymphoma in chickens, is poorly understood. Two different experiments were used to examine the potential role of particular cytokines in the pathogenesis and immune responses of MD. First, chicken embryo fibroblasts (CEF) were stimulated with lipopolysaccharide (LPS) and/or recombinant chicken interferon-gamma (rChIFN-gamma) and used to develop techniques for examining transcription of IFN-alpha, IFN-gamma, inducible nitric oxide synthase (iNOS), interleukin (IL)-1beta, IL-2, IL-6 and IL-8 by reverse transcription-polymerase chain reaction (RT-PCR). Addition of LPS and/or rChIFN-gamma resulted in the up-regulation of mRNA for iNOS, IL-1beta and IL-6, while IFN-gamma was up-regulated by LPS alone. IL-2 was down-regulated by the treatments. Second, to determine the effects of Marek's disease herpesvirus (MDV) infection on cytokine transcription in vivo, chickens were infected with MDV at 21 days of age and examined at 7 days post-infection (p.i.) (exp. 1) or were infected with MDV at 1 day of age and examined from 3 to 15 days p.i. (exp. 2). In MDV-infected chickens, IFN-gamma transcription was up-regulated as early as 3 days p.i. until the termination of the experiment at 15 days p.i., while iNOS and IL-1beta were up-regulated between 6 and 15 days p.i. Infection of 1-day-old chicks increased levels of mRNA for IFN-gamma and iNOS between 16- and 64-fold at 9 days p.i. These results suggest that IFN-gamma and iNOS may play an important role in the pathogenesis of MD.
Figures





References
-
- Calnek BW. Marek's disease – a model for herpesvirus oncology. CRC Crit Rev Microbiol. 1986;12:293. - PubMed
-
- Schat KA. Marek's disease: a model for protection against herpesvirus-induced tumors. Cancer Surveys. 1987;6:1. - PubMed
-
- Omar AR, Schat KA. Syngeneic Marek's disease virus (MDV)-specific cell-mediated immune responses against immediate early, late and unique MDV proteins. Virology. 1996;222:87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

