Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions
- PMID: 10810146
- PMCID: PMC139923
- DOI: 10.1105/tpc.12.5.721
Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions
Abstract
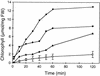
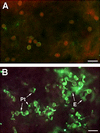
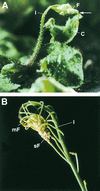
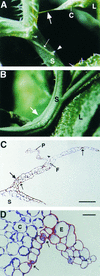
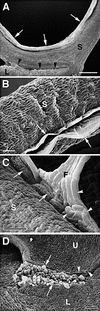
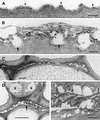
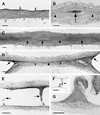
A major structural component of the cuticle of plants is cutin. Analysis of the function of cutin in vivo has been limited because no mutants with specific defects in cutin have been characterized. Therefore, transgenic Arabidopsis plants were generated that express and secrete a cutinase from Fusarium solani f sp pisi. Arabidopsis plants expressing the cutinase in the extracellular space showed an altered ultrastructure of the cuticle and an enhanced permeability of the cuticle to solutes. In addition, pollen could germinate on fully differentiated leaves of cutinase-expressing plants but not on control leaves. These differences coincided with strong postgenital organ fusions. The junctions of the fusions contained pectic polysaccharides. As fused organs grew apart from each other, organ deformations and protrusions of epidermal cells developed at positions with high mechanical stress. These results demonstrate that an intact cutin layer not only is important for plant-environment interactions but also prevents fusions between different plant organs and is therefore necessary for normal epidermal differentiation and organ formation.
Figures


References
-
- Baker, C.J., McCormick, S.L., and Batemann, D.F. (1982). Effects of purified cutin esterase upon the permeability and mechanical strength of cutin membranes. Phytopathology 72, 420–423.
-
- Baker, E.A. (1982). Chemistry and morphology of plant epicuticular waxes. In The Plant Cuticle, D.F. Cutler, K.L. Alvin, and C.E. Price, eds (London: Academic Press), pp. 139–162.
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. 316, 1194–1199. - PubMed
-
- Becraft, P.W., Stinard, P.S., and McCarty, D.R. (1996). CRINKLY4: A TNKR-like receptor kinase involved in epidermal maize differentiation. Science 273, 1406–1409. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

