Identification, purification, and molecular cloning of a putative plastidic glucose translocator
- PMID: 10810150
- PMCID: PMC139927
- DOI: 10.1105/tpc.12.5.787
Identification, purification, and molecular cloning of a putative plastidic glucose translocator
Abstract
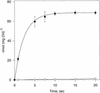
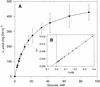
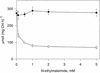
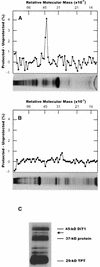
During photosynthesis, part of the fixed carbon is directed into the synthesis of transitory starch, which serves as an intermediate carbon storage facility in chloroplasts. This transitory starch is mobilized during the night. Increasing evidence indicates that the main route of starch breakdown proceeds by way of hydrolytic enzymes and results in glucose formation. This pathway requires a glucose translocator to mediate the export of glucose from the chloroplasts. We have reexamined the kinetic properties of the plastidic glucose translocator and, using a differential labeling procedure, have identified the glucose translocator as a component of the inner envelope membrane. Peptide sequence information derived from this protein was used to isolate cDNA clones encoding a putative plastidic glucose translocator from spinach, potato, tobacco, Arabidopsis, and maize. We also present the molecular characterization of a candidate for a hexose transporter of the plastid envelope membrane. This transporter, initially characterized more than 20 years ago, is closely related to the mammalian glucose transporter GLUT family and differs from all other plant hexose transporters that have been characterized to date.
Figures



References
-
- Baldwin, S.A., and Lienhard, G.E. (1989). Purification and reconstitution of glucose transporter from human erythrocytes. Methods Enzymol. 174, 39–50. - PubMed
-
- Bechtold, N., Ellis, G., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. 316, 1194–1199. - PubMed
-
- Beck, E. (1985). The degradation of transitory starch in chloroplasts. In Regulation of Carbon Partitioning in Photosynthetic Tissue, R.L. Heath and J. Preiss, eds (Baltimore, MD: Waverly), pp. 27–44.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

