Thr38 and Ser198 are Pto autophosphorylation sites required for the AvrPto-Pto-mediated hypersensitive response
- PMID: 10811617
- PMCID: PMC384356
- DOI: 10.1093/emboj/19.10.2257
Thr38 and Ser198 are Pto autophosphorylation sites required for the AvrPto-Pto-mediated hypersensitive response
Erratum in
- EMBO J 2000 Jun 15;19(12):3157
Abstract
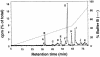
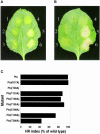
The tomato Pto kinase confers resistance to Pseudomonas syringae pv. tomato expressing the AvrPto protein. To elucidate the role of Pto autophosphorylation in disease resistance, eight sites autophosphorylated by Pto in vitro were identified by a combination of HPLC purification of tryptic phosphopeptides, MALDI-TOF/MS analysis and Edman degradation. Mutational analysis of the autophosphorylation sites revealed that Pto residues Thr38 and Ser198 are required for AvrPto-Pto- mediated elicitation of a hypersensitive response in the plant. Thr38, which is the main Pto autophosporylation site and is located outside the kinase catalytic domain, was also required for Pto kinase activity and its physical interaction with AvrPto, the Pti1 kinase and the transcription factor Pti4. Ser198, located in the Pto activation domain, was dispensable for kinase activity and for interaction with AvrPto. However, a mutation at this site resulted in altered Pto interactions with the Pti1 kinase and the Pto interactors of unknown function Pti3 and Pti10. These results suggest that autophosphorylation events at Pto Thr38 and Ser198 are required for signal transduction by Pto and participate in distinct molecular mechanisms.
Figures






References
-
- Baker B., Zambryski,P., Staskawicz,B. and Dinesh-Kumar,S.P. (1997) Signaling in plant–microbe interactions. Science, 276, 726–733. - PubMed
-
- Fish Gietzen K. and Virshup,D.M. (1999) Identification of inhibitory autophosphorylation sites in casein kinase Iε. J. Biol. Chem., 274, 32063–32070. - PubMed
-
- Frederick R.D., Thilmony,R.L., Sessa,G. and Martin,G.B. (1998) Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol. Cell, 2, 241–245. - PubMed
-
- Galan J.E. and Collmer,A. (1999) Type III secretion machines: bacterial devices for protein delivery into host cells. Science, 284, 1322–1328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

