The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue
- PMID: 10811626
- PMCID: PMC384370
- DOI: 10.1093/emboj/19.10.2351
The 2 A crystal structure of leucyl-tRNA synthetase and its complex with a leucyl-adenylate analogue
Abstract
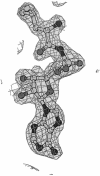
Leucyl-, isoleucyl- and valyl-tRNA synthetases are closely related large monomeric class I synthetases. Each contains a homologous insertion domain of approximately 200 residues, which is thought to permit them to hydrolyse ('edit') cognate tRNA that has been mischarged with a chemically similar but non-cognate amino acid. We describe the first crystal structure of a leucyl-tRNA synthetase, from the hyperthermophile Thermus thermophilus, at 2.0 A resolution. The overall architecture is similar to that of isoleucyl-tRNA synthetase, except that the putative editing domain is inserted at a different position in the primary structure. This feature is unique to prokaryote-like leucyl-tRNA synthetases, as is the presence of a novel additional flexibly inserted domain. Comparison of native enzyme and complexes with leucine and a leucyl- adenylate analogue shows that binding of the adenosine moiety of leucyl-adenylate causes significant conformational changes in the active site required for amino acid activation and tight binding of the adenylate. These changes are propagated to more distant regions of the enzyme, leading to a significantly more ordered structure ready for the subsequent aminoacylation and/or editing steps.
Figures
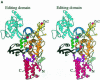
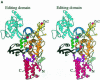
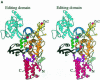
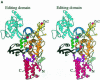






References
-
- Asahara H., Himeno,H., Tamura,K., Hasegawa,T., Watanabe,K. and Shimizu,M. (1993) Recognition nucleotides of Escherichia coli tRNALeu and its elements facilitating discrimination from tRNASer and tRNATyr. J. Mol. Biol., 231, 219–229. - PubMed
-
- Brünger A.T. et al. (1998) Crystallographic and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
-
- Carter C.W. Jr (1993) Cognition, mechanism and evolutionary relationships in aminoacyl-tRNA synthetases. Annu. Rev. Biochem., 62, 715–748. - PubMed
-
- Collaborative Computational Project Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

