Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations
- PMID: 10811719
- PMCID: PMC2269938
- DOI: 10.1111/j.1469-7793.2000.t01-4-00001.x
Receptors, gephyrin and gephyrin-associated proteins: novel insights into the assembly of inhibitory postsynaptic membrane specializations
Abstract
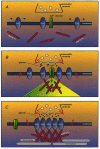
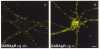
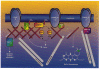
The synaptic localization of ion channel receptors is essential for efficient synaptic trans-mission and the precise regulation of diverse neuronal functions, such as signal integration and synaptic plasticity. Emerging evidence points to an important role of cytoskeleton-associated proteins that assemble receptors and components of the subsynaptic machinery at postsynaptic membrane specializations. This article reviews interactions of inhibitory postsynaptic neurotransmitter receptors with the receptor anchoring protein gephyrin and intracellular components involved in downstream signalling and/or control of signal transduction processes. The presently available data suggest a central synaptic organizer function for gephyrin in inhibitory postsynaptic membrane assembly and stabilization.
Figures
References
-
- Alberts AS, Bouquin N, Johnston LH, Treisman R. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. Journal of Biological Chemistry. 1998;273:8616–8622. - PubMed
-
- Araki T, Yamano M, Murakami T, Wanaka A, Betz H, Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988;25:613–624. - PubMed
-
- Bechade C, Colin I, Kirsch J, Betz H, Triller A. Expression of glycine receptor α subunits and gephyrin in cultured spinal neurons. European Journal of Neuroscience. 1996;8:429–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

