An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells
- PMID: 10811732
- PMCID: PMC2269923
- DOI: 10.1111/j.1469-7793.2000.00135.x
An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells
Abstract
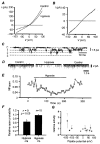
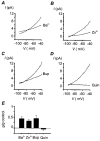
The biophysical and pharmacological properties of an oxygen-sensitive background K+ current in rat carotid body type-I cells were investigated and compared with those of recently cloned two pore domain K+ channels. Under symmetrical K+ conditions the oxygen-sensitive whole cell K+ current had a linear dependence on voltage indicating a lack of intrinsic voltage sensitivity. Single channel recordings identified a K+ channel, open at resting membrane potentials, that was inhibited by hypoxia. This channel had a single channel conductance of 14 pS, flickery kinetics and showed little voltage sensitivity except at extreme positive potentials. Oxygen-sensitive current was inhibited by 10 mM barium (57% inhibition), 200 microM zinc (53% inhibition), 200 microM bupivacaine (55% inhibition) and 1 mM quinidine (105 % inhibition). The general anaesthetic halothane (1.5%) increased the oxygen-sensitive K+ current (by 176%). Halothane (3 mM) also stimulated single channel activity in inside-out patches (by 240%). Chloroform had no effect on background K+ channel activity. Acidosis (pH 6.4) inhibited the oxygen-sensitive background K+ current (by 56%) and depolarised type-I cells. The pharmacological and biophysical properties of the background K+ channel are, therefore, analogous to those of the cloned channel TASK-1. Using in situ hybridisation TASK-1 mRNA was found to be expressed in type-I cells. We conclude that the oxygen- and acid-sensitive background K+ channel of carotid body type-I cells is likely to be an endogenous TASK-1-like channel.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

