Structural transitions at microtubule ends correlate with their dynamic properties in Xenopus egg extracts
- PMID: 10811818
- PMCID: PMC2174571
- DOI: 10.1083/jcb.149.4.767
Structural transitions at microtubule ends correlate with their dynamic properties in Xenopus egg extracts
Abstract
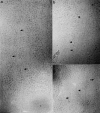
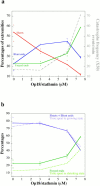
Microtubules are dynamically unstable polymers that interconvert stochastically between growing and shrinking states by the addition and loss of subunits from their ends. However, there is little experimental data on the relationship between microtubule end structure and the regulation of dynamic instability. To investigate this relationship, we have modulated dynamic instability in Xenopus egg extracts by adding a catastrophe-promoting factor, Op18/stathmin. Using electron cryomicroscopy, we find that microtubules in cytoplasmic extracts grow by the extension of a two- dimensional sheet of protofilaments, which later closes into a tube. Increasing the catastrophe frequency by the addition of Op18/stathmin decreases both the length and frequency of the occurrence of sheets and increases the number of frayed ends. Interestingly, we also find that more dynamic populations contain more blunt ends, suggesting that these are a metastable intermediate between shrinking and growing microtubules. Our results demonstrate for the first time that microtubule assembly in physiological conditions is a two-dimensional process, and they suggest that the two-dimensional sheets stabilize microtubules against catastrophes. We present a model in which the frequency of catastrophes is directly correlated with the structural state of microtubule ends.
Figures



References
-
- Amos L. The microtubule lattice20 years on. Trends Cell Biol. 1995;5:48–51. - PubMed
-
- Bayley P.M., Schilstra M.J., Martin S.R. Microtubule dynamic instabilitynumerical simulation of microtubule transition properties using a lateral cap model. J. Cell Sci. 1990;95:33–48. - PubMed
-
- Belmont L.D., Mitchison T.J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. - PubMed
-
- Belmont L.D., Hyman A.A., Sawin K.E., Mitchison T.J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. - PubMed
-
- Bornens M., Paintrand M., Berges J., Marty M.C., Karsenti E. Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskeleton. 1987;8:238–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

