HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death
- PMID: 10811848
- PMCID: PMC315465
- DOI: 10.1172/JCI8707
HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death
Retraction in
-
Retraction. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death.J Clin Invest. 2010 Sep;120(9):3401. doi: 10.1172/jci8707r1. J Clin Invest. 2010. PMID: 20830824 Free PMC article. No abstract available.
Expression of concern in
-
Expression of concern.J Clin Invest. 2008 May;118(5):1974. doi: 10.1172/JCI8707EX1. J Clin Invest. 2008. PMID: 18451992 Free PMC article. No abstract available.
Abstract
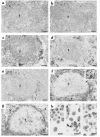
We have tracked the in vivo migration and have identified in vivo correlates of cytotoxic T-lymphocyte (CTL) activity in HIV-seropositive subjects infused with autologous gene-marked CD8(+) HIV-specific CTL. The number of circulating gene-marked CTL ranged from 1.6 to 3.5% shortly after infusion to less than 0.5% 2 weeks later. Gene-marked CTL were present in the lymph node at 4.5- to 11-fold excess and colocalized within parafollicular regions of the lymph node adjacent to cells expressing HIV tat fusion transcripts, a correlate of virus replication. The CTL clones expressed the CCR5 receptor and localized among HIV-infected cells expressing the ligands MIP-1alpha and MIP-1beta, CC-chemokines produced at sites of virus replication. Aggregates of apoptotic cells and cells expressing granzyme-B localized within these same sites. In contrast, lymph node sections from untreated HIV-seropositive subjects, all with significant viral burden (> 50,000 HIV RNA copies/mL plasma), showed no CC-chemokine expression and exhibited only sporadic and randomly distributed cells expressing granzymes and/or apoptotic cells. These studies show that the infused CTL specifically migrate to sites of HIV replication and retain their antigen-specific cytolytic potential. Moreover, these studies provide a methodology that will facilitate studies of both the magnitude and functional phenotype of Ag-specific CD8(+) T cells in vivo.
Figures


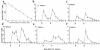


Comment in
-
Proving HIV-1 immunity: new tools offer new opportunities.J Clin Invest. 2000 May;105(10):1333-4. doi: 10.1172/JCI10049. J Clin Invest. 2000. PMID: 10811838 Free PMC article. Review. No abstract available.
-
Findings of misconduct in science.NIH Guide Grants Contracts (Bethesda). 2010 May 14:NOT-OD-10-095. NIH Guide Grants Contracts (Bethesda). 2010. PMID: 20486276 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

