Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia
- PMID: 10811849
- PMCID: PMC315457
- DOI: 10.1172/JCI4546
Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia
Abstract
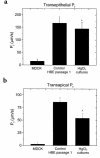
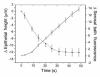
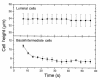
Current hypotheses describing the function of normal airway surface liquid (ASL) in lung defense are divergent. One theory predicts that normal airways regulate ASL volume by modulating the flow of isosmotic fluid across the epithelium, whereas an alternative theory predicts that ASL is normally hyposmotic. These hypotheses predict different values for the osmotic water permeability (P(f)) of airway epithelia. We measured P(f) of cultures of normal and cystic fibrosis (CF) airway epithelia that, like the native tissue, contain columnar cells facing the lumen and basal cells that face a basement membrane. Xz laser scanning confocal microscopy recorded changes in epithelial height and transepithelial volume flow in response to anisosmotic challenges. With luminal hyperosmotic challenges, transepithelial and apical membrane P(f) are relatively high for both normal and CF airway epithelia, consistent with an isosmotic ASL. Simultaneous measurements of epithelial cell volume and transepithelial water flow revealed that airway columnar epithelial cells behave as osmometers whose volume is controlled by luminal osmolality. Basal cell volume did not change in these experiments. When the serosal side of the epithelium was challenged with hyperosmotic solutions, the basal cells shrank, whereas the lumen-facing columnar cells did not. We conclude that (a) normal and CF airway epithelia have relatively high water permeabilities, consistent with the isosmotic ASL theory, and the capacity to restore water on airway surfaces lost by evaporation, and (b) the columnar cell basolateral membrane and tight junctions limit transepithelial water flow in this tissue.
Figures

Comment in
-
Airway plumbing.J Clin Invest. 2000 May;105(10):1343-4. doi: 10.1172/JCI10088. J Clin Invest. 2000. PMID: 10811841 Free PMC article. Review. No abstract available.
References
-
- Boucher RC. Human airway ion transport. Part two. Am J Respir Crit Care Med. 1994;150:581–593. - PubMed
-
- Quinton PM. Viscosity versus composition in airway pathology. Am J Respir Crit Care Med. 1994;149:6–7. - PubMed
-
- Quinton PM. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990;4:2709–2717. - PubMed
-
- Stutts MJ, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

