Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation
- PMID: 10811855
- PMCID: PMC315469
- DOI: 10.1172/JCI9523
Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation
Abstract
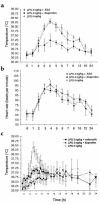
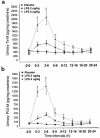
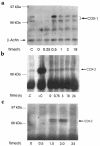
To examine the role of cyclooxygenase (COX) isozymes in prostaglandin formation and oxidant stress in inflammation, we administered to volunteer subjects placebo or bolus injections of lipopolysaccharide (LPS), which caused a dose-dependent increase in temperature, heart rate, and plasma cortisol. LPS caused also dose-dependent elevations in urinary excretion of 2,3-dinor 6-keto PGF(1alpha) (PGI-M) and 11-dehydro thromboxane B(2) (Tx-M). Platelet COX-1 inhibition by chronic administration of low-dose aspirin before LPS did not alter the symptomatic and febrile responses to LPS, but the increment in urinary PGI-M and Tx-M were both partially depressed. Pretreatment with ibuprofen, a nonspecific COX inhibitor, attenuated the febrile and systemic response to LPS and inhibited prostanoid biosynthesis. Both celecoxib, a selective COX-2 inhibitor, and ibuprofen attenuated the pyrexial, but not the chronotropic, response to LPS. Experimental endotoxemia caused differential expression of the COX isozymes in monocytes and polymorphonuclear leucocytes ex vivo. LPS also increased urinary iPF(2alpha)-III, iPF(2alpha)-VI, and 8,12-iso-iPF(2alpha)-VI, isoprostane (iP) indices of lipid peroxidation, and none of the drugs blunted this response. These studies indicate that (a) although COX-2 predominates, both COX isozymes are induced and contribute to the prostaglandin response to LPS in humans; (b) COX activation contributes undetectably to lipid peroxidation induced by LPS; and (c) COX-2, but not COX-1, contributes to the constitutional response to LPS in humans.
Figures


References
-
- FitzGerald, G.A. 1992. Prostaglandins and related compounds. In Cecil textbook of medicine. 19th edition. J.B. Wyngaarden, L.H. Smith, and J.C. Bennett, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 1271–1276.
-
- Dubois RN, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. - PubMed
-
- Smith WL. Prostanoid biosynthesis and mechanisms of action. Am J Physiol. 1992;263:F181–F191. - PubMed
-
- Funk CD, Funk LB, Kennedy ME, Pong AS, FitzGerald GA. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. FASEB J. 1991;5:2304–2312. - PubMed
-
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–33160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

