Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo
- PMID: 10811860
- PMCID: PMC2193147
- DOI: 10.1084/jem.191.10.1661
Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo
Abstract
The fungus Candida albicans behaves as a commensal as well as a true pathogen of areas highly enriched in dendritic cells, such as skin and mucosal surfaces. The ability of the fungus to reversibly switch between unicellular yeast to filamentous forms is thought to be important for virulence. However, whether it is the yeast or the hyphal form that is responsible for pathogenicity is still a matter of debate. Here we show the interaction, and consequences, of different forms of C. albicans with dendritic cells. Immature myeloid dendritic cells rapidly and efficiently phagocytosed both yeasts and hyphae of the fungus. Phagocytosis occurred through different phagocytic morphologies and receptors, resulting in phagosome formation. However, hyphae escaped the phagosome and were found lying free in the cytoplasm of the cells. In vitro, ingestion of yeasts activated dendritic cells for interleukin (IL)-12 production and priming of T helper type 1 (Th1) cells, whereas ingestion of hyphae inhibited IL-12 and Th1 priming, and induced IL-4 production. In vivo, generation of antifungal protective immunity was induced upon injection of dendritic cells ex vivo pulsed with Candida yeasts but not hyphae. The immunization capacity of yeast-pulsed dendritic cells was lost in the absence of IL-12, whereas that of hypha-pulsed dendritic cells was gained in the absence of IL-4. These results indicate that dendritic cells fulfill the requirement of a cell uniquely capable of sensing the two forms of C. albicans in terms of type of immune responses elicited. By the discriminative production of IL-12 and IL-4 in response to the nonvirulent and virulent forms of the fungus, dendritic cells appear to meet the challenge of Th priming and education in C. albicans saprophytism and infections.
Figures
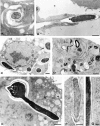





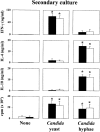

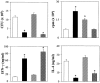
References
-
- Edwards J.E. Invasive Candida infectionsevolution of a fungal pathogen. N. Engl. J. Med. 1991;324:1060–1062. - PubMed
-
- Romani L., Puccetti P., Bistoni F. Biological role of helper T-cell subsets in candidiasis. Chem. Immunol. 1996;63:113–137. - PubMed
-
- Romani L. The T cell response to fungi. Curr. Opin. Immunol. 1997;9:484–490. - PubMed
-
- Romani L. Immunity to Candida albicansTh1, Th2 and beyond. Curr. Opin. Microbiol. 1999;2:363–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

