Protein oxidation in response to increased transcriptional or translational errors
- PMID: 10811907
- PMCID: PMC18504
- DOI: 10.1073/pnas.100422497
Protein oxidation in response to increased transcriptional or translational errors
Abstract
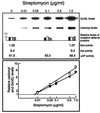
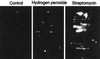
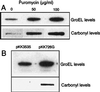
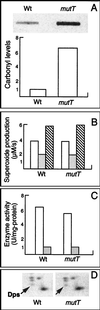
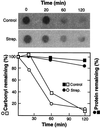
In this study, we show a correlation between synthesis of aberrant proteins and their oxidative modification. The level of aberrant proteins was elevated in Escherichia coli cultures by decreasing transcriptional or translational fidelity using specific mutations or drugs. Protein carbonylation, an oxidative modification, increased in parallel to the induction of the heat shock chaperone GroEL. As the protein turnover rates and level of intracellular oxidative stress remained unchanged, it appears that carbonylation results from the increased susceptibility of the misfolded proteins. These studies show that the cellular protein oxidation is not limited only by available reactive oxygen species, but by the levels of aberrant proteins. Thus, protein oxidation seen in aging cells may be the consequence also of reduced transcriptional/translational fidelity, and protein structures appear to have evolved to minimize oxidative damage. In addition, we discuss the possibility that carbonylation, being an unrepairable protein modification, may serve as a tagging system to shunt misfolded proteins between pathways of refolding by chaperones or the proteolytic apparatus.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

