Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells
- PMID: 10816511
- PMCID: PMC97642
- DOI: 10.1128/IAI.68.6.3554-3563.2000
Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells
Abstract
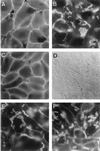
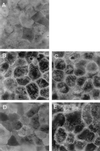
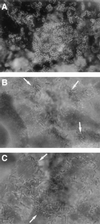
The Afa/Dr family of diffusely adhering Escherichia coli (Afa/Dr DAEC) includes bacteria expressing afimbrial adhesins (AFA), Dr hemagglutinin, and fimbrial F1845 adhesin. We show that infection of human intestinal Caco-2/TC7 cells by the Afa/Dr DAEC strains C1845 and IH11128 is followed by clustering of CD55 around adhering bacteria. Mapping of CD55 epitopes involved in CD55 clustering by Afa/Dr DAEC was conducted using CD55 deletion mutants expressed by stable transfection in CHO cells. Deletion in the short consensus repeat 1 (SCR1) domain abolished Afa/Dr DAEC-induced CD55 clustering. In contrast, deletion in the SCR4 domain does not modify Afa/Dr DAEC-induced CD55 clustering. We show that the brush border-associated glycosylphosphatidylinositol (GPI)-anchored protein CD66e (carcinoembryonic antigen) is recruited by the Afa/Dr DAEC strains C1845 and IH11128. This conclusion is based on the observations that (i) infection of Caco-2/TC7 cells by Afa/Dr DAEC strains is followed by clustering of CD66e around adhering bacteria and (ii) Afa/Dr DAEC strains bound efficiently to stably transfected HeLa cells expressing CD66e, accompanied by CD66e clustering around adhering bacteria. Inhibition assay using monoclonal antibodies directed against CD55 SCR domains, and polyclonal anti-CD55 and anti-CD66e antibodies demonstrate that CD55 and CD66e function as a receptors for the C1845 and IH11128 bacteria. Moreover, using structural draE gene mutants, we found that a mutant in which cysteine replaced aspartic acid at position 54 displayed conserved binding capacity but failed to induce CD55 and CD66e clustering. Taken together, these data give new insights into the mechanisms by which Afa/Dr DAEC induces adhesin-dependent cross talk in the human polarized intestinal epithelial cells by mobilizing brush border-associated GPI-anchored proteins known to function as transducing molecules.
Figures


References
-
- Abraham S N, Jonsson A-B, Normark S. Fimbriae-mediated host-pathogen cross-talk. Curr Opin Microbiol. 1998;1:75–81. - PubMed
-
- Anderson R G W. The caveola membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
-
- Bernet-Camard M F, Coconnier M H, Hudault S, Servin A L. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect Immun. 1996;64:1818–1828. - PMC - PubMed
-
- Bilge S S, Apostol J M, Jr, Fullner K J, Moseley S L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993;7:993–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

