Cooperation between reactive oxygen and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages
- PMID: 10816516
- PMCID: PMC97647
- DOI: 10.1128/IAI.68.6.3587-3593.2000
Cooperation between reactive oxygen and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages
Abstract
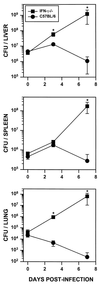
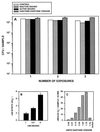
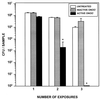
Rhodococcus equi is a facultative intracellular bacterium of macrophages which can infect immunocompromised humans and young horses. In the present study, we examine the mechanism of host defense against R. equi by using a murine model. We show that bacterial killing is dependent upon the presence of gamma interferon (IFN-gamma), which activates macrophages to produce reactive nitrogen and oxygen intermediates. These two radicals combine to form peroxynitrite (ONOO(-)), which kills R. equi. Mice deficient in the production of either the high-output nitric oxide pathway (iNOS(-/-)) or the oxidative burst (gp91(phox-/-)) are more susceptible to lethal R. equi infection and display higher bacterial burdens in their livers, spleens, and lungs than wild-type mice. These in vivo observations, which implicate both nitric oxide (NO) and superoxide (O(2)(-)) in bacterial killing, were reexamined in cell-free radical-generating assays. In these assays, R. equi remains fully viable following prolonged exposure to high concentrations of either nitric oxide or superoxide, indicating that neither compound is sufficient to mediate bacterial killing. In contrast, brief exposure of bacteria to ONOO(-) efficiently kills virulent R. equi. The intracellular killing of bacteria in vitro by activated macrophages correlated with the production of ONOO(-) in situ. Inhibition of nitric oxide production by activated macrophages by using N(G)-monomethyl-L-arginine blocks their production of ONOO(-) and weakens their ability to control rhodococcal replication. These studies indicate that peroxynitrite mediates the intracellular killing of R. equi by IFN-gamma-activated macrophages.
Figures




Similar articles
-
Nitric oxide-mediated intracellular growth restriction of pathogenic Rhodococcus equi can be prevented by iron.Infect Immun. 2011 May;79(5):2098-111. doi: 10.1128/IAI.00983-10. Epub 2011 Mar 7. Infect Immun. 2011. PMID: 21383050 Free PMC article.
-
Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro.J Exp Med. 2000 Jul 17;192(2):227-36. doi: 10.1084/jem.192.2.227. J Exp Med. 2000. PMID: 10899909 Free PMC article.
-
Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani.J Exp Med. 1999 Feb 15;189(4):741-6. doi: 10.1084/jem.189.4.741. J Exp Med. 1999. PMID: 9989990 Free PMC article.
-
Pathogenesis and virulence of Rhodococcus equi.Vet Microbiol. 1997 Jun 16;56(3-4):257-68. doi: 10.1016/s0378-1135(97)00094-1. Vet Microbiol. 1997. PMID: 9226840 Review.
-
NADPH oxidase, Nramp1 and nitric oxide synthase 2 in the host antimicrobial response.Rev Immunogenet. 2000;2(3):387-415. Rev Immunogenet. 2000. PMID: 11256747 Review.
Cited by
-
Mycoredoxins Are Required for Redox Homeostasis and Intracellular Survival in the Actinobacterial Pathogen Rhodococcus equi.Antioxidants (Basel). 2019 Nov 15;8(11):558. doi: 10.3390/antiox8110558. Antioxidants (Basel). 2019. PMID: 31731720 Free PMC article.
-
Host-directed therapy in foals can enhance functional innate immunity and reduce severity of Rhodococcus equi pneumonia.Sci Rep. 2021 Jan 28;11(1):2483. doi: 10.1038/s41598-021-82049-y. Sci Rep. 2021. PMID: 33510265 Free PMC article.
-
Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens.Biofactors. 2014 Mar-Apr;40(2):215-25. doi: 10.1002/biof.1150. Epub 2013 Nov 26. Biofactors. 2014. PMID: 24281946 Free PMC article. Review.
-
Nitric oxide-mediated intracellular growth restriction of pathogenic Rhodococcus equi can be prevented by iron.Infect Immun. 2011 May;79(5):2098-111. doi: 10.1128/IAI.00983-10. Epub 2011 Mar 7. Infect Immun. 2011. PMID: 21383050 Free PMC article.
-
Macrophage effector responses of horses are influenced by expression of CD154.Vet Immunol Immunopathol. 2016 Nov 1;180:40-44. doi: 10.1016/j.vetimm.2016.08.001. Epub 2016 Aug 26. Vet Immunol Immunopathol. 2016. PMID: 27692094 Free PMC article.
References
-
- Assreuy J, Cunha F Q, Epperlein M, Noronha-Dutra A, O'Donell C A, Liew F Y, Moncada S. Production of nitric oxide and superoxide by activated macrophages and killing of Leishmania major. Eur J Immunol. 1994;24:672–676. - PubMed
-
- Babior B M, Curnette J T, Kipnes R S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975;85:235–244. - PubMed
-
- Beckman J S, Ischiropoulos H, Zhu L, van der Woerd M, Smith C, Chen J, Harrison J, Martin J C, Tsai M H. Kinetics of superoxide dismutase and iron catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992;298:431–437. - PubMed
-
- Brunelli L, Crow J P, Beckman J S. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch Biochem Biophys. 1995;316:327–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

