Gut peptide receptor expression in human pancreatic cancers
- PMID: 10816627
- PMCID: PMC1421073
- DOI: 10.1097/00000658-200006000-00008
Gut peptide receptor expression in human pancreatic cancers
Abstract
Objective: To determine the prevalence of gastrointestinal (GI) peptide receptor expression in pancreatic cancers, and to further assess signaling mechanisms regulating neurotensin (NT)-mediated pancreatic cancer growth.
Summary background data: Pancreatic cancer remains one of the leading causes of GI cancer death; novel strategies for the early detection and treatment of these cancers is required. Previously, the authors have shown that NT, an important GI hormone, stimulates the proliferation of an NT receptor (NTR)-positive pancreatic cancer.
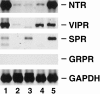
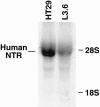
Methods: A total of 26 human pancreatic adenocarcinomas, obtained after resection, and 5 pancreatic cancer xenografts were analyzed for expression of NTR, vasoactive intestinal peptide receptor (VIPR), substance P receptor (SPR), and gastrin-releasing peptide receptor (GRPR). In addition, NTR expression, [Ca2+]i mobilization, and growth in response to NT was determined in L3.6, a metastatic pancreatic cancer cell line.
Results: Neurotensin receptor was expressed in 88% of the surgical specimens examined and all five of the pancreatic cancer xenografts. In contrast, VIPR, SPR, and GRPR expression was detected in 31%, 27%, and 8% of pancreatic cancers examined, respectively. Expression of NTR, functionally coupled to the Ca2+ signaling pathway, was identified in L3.6 cells; treatment with NT (10 micromol/L) stimulated proliferation of these cells.
Conclusions: The authors demonstrated NTR expression in most of the pancreatic adenocarcinomas examined. In contrast, VIPR, SPR, and GRPR expression was detected in fewer of the pancreatic cancers. The expression of NTR and other peptide receptors suggests the potential role of endocrine manipulation in the treatment of these cancers. Further, the presence of GI receptors may provide for targeted chemotherapy or radiation therapy or in vivo scintigraphy for early detection.
Figures



References
-
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin 1999; 49:8–31. - PubMed
-
- Royce ME, Pazdur R. Novel chemotherapeutic agents for gastrointestinal cancers. Curr Opin Oncol 1999; 11:299–304. - PubMed
-
- Green S, Furr B. Prospects for the treatment of endocrine-responsive tumours. Endocr Relat Cancer 1999; 6:349–371. - PubMed
-
- Townsend CM Jr, Singh P, Thompson JC. Hormonal effects on gastrointestinal cancer growth. In: Etievant C, Cros J, Rustum YM, eds. New Concepts in Cancer: Metastasis, Oncogenes and Growth Factors. London: MacMillan Press; 1990: 208–217.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

