The Legionella (Fluoribacter) gormanii metallo-beta-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 beta-lactamases
- PMID: 10817705
- PMCID: PMC89909
- DOI: 10.1128/AAC.44.6.1538-1543.2000
The Legionella (Fluoribacter) gormanii metallo-beta-lactamase: a new member of the highly divergent lineage of molecular-subclass B3 beta-lactamases
Abstract
A metallo-beta-lactamase determinant was cloned from a genomic library of Legionella (Fluoribacter) gormanii ATCC 33297(T) constructed in the plasmid vector pACYC184 and transformed into Escherichia coli DH5alpha, by screening for clones showing a reduced susceptibility to imipenem. The product of the cloned determinant, named FEZ-1, contains a 30-kDa polypeptide and exhibits an isoelectric pH of 7.6. Sequencing revealed that FEZ-1 is a molecular-class B beta-lactamase which shares the closest structural similarity (29.7% of identical residues) with the L1 enzyme of Stenotrophomonas maltophilia, being a new member of the highly divergent subclass B3 lineage. All the residues that in L1 are known to be directly or indirectly involved in coordination of the zinc ions were found to be conserved also in FEZ-1, suggesting that the geometry of zinc coordination in the active site of the latter enzyme is identical to that of L1. Unlike L1, however, FEZ-1 appeared to be monomeric in gel permeation chromatography experiments and exhibited a distinctive substrate specificity with a marked preference for cephalosporins and meropenem. The properties of FEZ-1 overall resembled those of a beta-lactamase previously purified from the same strain of L. gormanii (T. Fujii, K. Sato, K. Miyata, M. Inoue, and S. Mitsuhashi, Antimicrob. Agents Chemother. 29:925-926, 1986) and are as yet unique among class B enzymes, reinforcing the notion that considerable functional heterogeneity can be encountered among members of this class. A system for overexpression of the bla(FEZ-1) gene in E. coli, based on the T7 phage promoter, was also developed.
Figures




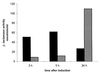
 , activity in the
culture supernatant.
, activity in the
culture supernatant.References
-
- Baxter I A, Lambert P A. Isolation and partial purification of a carbapenem-hydrolysing metallo-β-lactamase from Pseudomonas cepacia. FEMS Microbiol Lett. 1994;122:251–256. - PubMed
-
- Bellais S, Leotard S, Poirel L, Naas T, Nordmann P. Molecular characterization of a carbapenem-hydrolysing β-lactamase from Chryseobacterium (Flavobacterium) indologenes. FEMS Microbiol Lett. 1999;171:127–132. - PubMed
-
- Brenner D J, Feeley J C, Weaver R E. Family VII. Legionellaceae. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The William & Wilkins Co.; 1984. p. 279.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

