In vivo antimalarial activity of the beta-carboline alkaloid manzamine A
- PMID: 10817722
- PMCID: PMC89926
- DOI: 10.1128/AAC.44.6.1645-1649.2000
In vivo antimalarial activity of the beta-carboline alkaloid manzamine A
Abstract
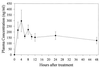
Manzamine A, a beta-carboline alkaloid present in several marine sponge species, inhibits the growth of the rodent malaria parasite Plasmodium berghei in vivo. More than 90% of the asexual erythrocytic stages of P. berghei were inhibited after a single intraperitoneal injection of manzamine A into infected mice. A remarkable aspect of manzamine A treatment is its ability to prolong the survival of highly parasitemic mice, with 40% recovery 60 days after a single injection. Oral administration of an oil suspension of manzamine A also produced significant reductions in parasitemia. The plasma manzamine A concentration peaked 4 h after injection and remained high even at 48 h. Morphological changes of P. berghei were observed 1 h after treatment of infected mice. (-)-8-Hydroxymanzamine A also displayed antimalarial activity, whereas manzamine F, a ketone analog of manzamine A, did not. Our results suggest that manzamine A and (-)-8-hydroxymanzamine A are promising new antimalarial agents.
Figures
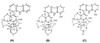

References
-
- Ager A L., Jr . Rodent malaria models. In: Peters W, Richards W H G, editors. Antimalarial drugs. I. Biological background, experimental methods and drug resistance. Berlin, Germany: Springer-Verlag; 1984. pp. 225–264.
-
- Cox F E G. Major animal models in malarial research: rodent. In: Wernsdorfer W H, McGregor I, editors. Malaria. Principles and practice of malariology. Edinburgh, Scotland: Churchill Livingstone, Ltd.; 1988. pp. 1503–1543.
-
- Edrada R A, Proksch P, Wray V, Witte L, Muller W E G, Van Soest R W M. Four new bioactive manzamine-type alkaloids from the Philippine marine sponge Xestospongia ashmorica. J Nat Prod. 1996;59:1056–1060. - PubMed
-
- El Sayed K A, Dunbar D C, Goins D K, Cordova C R, Perry T L, Wesson K J, Sanders S C, Janus S A, Hamann M T. The marine environment: a resource for prototype antimalarial agents. J Nat Tox. 1996;5:261–285.
-
- Heiffer M H, Davidson D E, Jr, Korte D W., Jr . Preclinical testing. In: Peters W, Richards W H G, editors. Antimalarial drugs. I. Biological background, experimental methods and drug resistance. Berlin, Germany: Springer-Verlag; 1984. pp. 351–373.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

