Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA)
- PMID: 10818025
- PMCID: PMC27379
- DOI: 10.1136/bmj.320.7246.1368
Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA)
Abstract
Objective: To examine the benefits of adding salmeterol compared with increasing dose of inhaled corticosteroids.
Design: Systematic review of randomised, double blind clinical trials. Independent data extraction and validation with summary data from study reports and manuscripts. Fixed and random effects analyses.
Setting: EMBASE, Medline, and GlaxoWellcome internal clinical study registers.
Main outcome measures: Efficacy and exacerbations.
Results: Among 2055 trials of treatment with salmeterol, there were nine parallel group trials of >/=12 weeks with 3685 symptomatic patients aged >/=12 years taking inhaled steroid in primary or secondary care. Compared with response to increased steroids, in patients receiving salmeterol morning peak expiratory flow was greater at three months (difference 22.4 (95% confidence interval 15.0 to 30.0) litre/min, P<0.001) and six months (27.7 (19.0 to 36.4) litre/min, P<0.001). Forced expiratory volume in one second (FEV(1)) was also increased at three months (0.10 (0.04 to 0.16) litres, P<0.001) and six months (0.08 (0.02 to 0.14) litres, P<0.01), as were mean percentage of days and nights without symptoms (three months: days-12% (9% to 15%), nights-5% (3% to 7%); six months: days-15% (12% to 18%), nights-5% (3% to 7%); all P<0.001) and mean percentage of days and nights without need for rescue treatment (three months: days-17% (14% to 20%), nights-9% (7% to 11%); six months: days-20% (17 to 23%), nights-8% (6% to 11%); all P<0.001). Fewer patients experienced any exacerbation with salmeterol (difference 2.73% (0.43% to 5.04%), P=0. 02), and the proportion of patients with moderate or severe exacerbations was also lower (2.42% (0.24% to 4.60%), P=0.03).
Conclusions: Addition of salmeterol in symptomatic patients aged 12 and over on low to moderate doses of inhaled steroid gives improved lung function and increased number of days and nights without symptoms or need for rescue treatment with no increase in exacerbations of any severity.
Figures

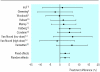
Comment in
-
Meta-analysis of increased inhaled steroid or addition of salmeterol in asthma. Greening et al's study should not have been included.BMJ. 2000 Oct 21;321(7267):1017; author reply 1017-8. BMJ. 2000. PMID: 11039980 No abstract available.
-
Meta-analysis of increased inhaled steroid or addition of salmeterol in asthma. Study should have been more thorough.BMJ. 2000 Oct 21;321(7267):1017-8. BMJ. 2000. PMID: 11039981 No abstract available.
-
Meta-analysis of increased inhaled steroid or addition of salmeterol in asthma. Researchers can learn from industry based reporting standards.BMJ. 2000 Oct 21;321(7267):1016-7; author reply 1017-8. BMJ. 2000. PMID: 11203208 Free PMC article. No abstract available.
References
-
- British Guidelines on Asthma Management. 1995 review and position statement. Thorax. 1997;52(suppl 1):1–21. - PubMed
-
- Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroids in asthma patients. Lancet. 1994;344:523–529. - PubMed
-
- Pauwels RA, Lofdahl C-G, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. New Engl J Med. 1997;337:1405–1411. - PubMed
-
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7:177–188. - PubMed
