Identification of proteins in the postsynaptic density fraction by mass spectrometry
- PMID: 10818142
- PMCID: PMC6772646
- DOI: 10.1523/JNEUROSCI.20-11-04069.2000
Identification of proteins in the postsynaptic density fraction by mass spectrometry
Abstract
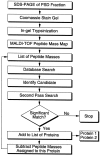
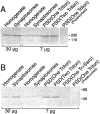
Our understanding of the organization of postsynaptic signaling systems at excitatory synapses has been aided by the identification of proteins in the postsynaptic density (PSD) fraction, a subcellular fraction enriched in structures with the morphology of PSDs. In this study, we have completed the identification of most major proteins in the PSD fraction with the use of an analytical method based on mass spectrometry coupled with searching of the protein sequence databases. At least one protein in each of 26 prominent protein bands from the PSD fraction has now been identified. We found 7 proteins not previously known to be constituents of the PSD fraction and 24 that had previously been associated with the PSD by other methods. The newly identified proteins include the heavy chain of myosin-Va (dilute myosin), a motor protein thought to be involved in vesicle trafficking, and the mammalian homolog of the yeast septin protein cdc10, which is important for bud formation in yeast. Both myosin-Va and cdc10 are threefold to fivefold enriched in the PSD fraction over brain homogenates. Immunocytochemical localization of myosin-Va in cultured hippocampal neurons shows that it partially colocalizes with PSD-95 at synapses and is also diffusely localized in cell bodies, dendrites, and axons. Cdc10 has a punctate distribution in cell bodies and dendrites, with some of the puncta colocalizing with PSD-95. The results support a role for myosin-Va in transport of materials into spines and for septins in the formation or maintenance of spines.
Figures




References
-
- Adam G, Matus A. Role of actin in the organization of brain postsynaptic densities. Brain Res Mol Brain Res. 1996;43:246–250. - PubMed
-
- Benfenati F, Valtorta F, Rubenstein JL, Gorelick FS, Greengard P, Czernik AJ. Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding-protein for synapsin I. Nature. 1992;359:417–420. - PubMed
-
- Bessman SP, Carpenter CL. The creatine-creatine phosphate energy shuttle. Annu Rev Biochem. 1985;54:831–862. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
