Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia
- PMID: 10818143
- PMCID: PMC6772642
- DOI: 10.1523/JNEUROSCI.20-11-04081.2000
Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia
Abstract
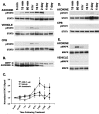
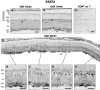
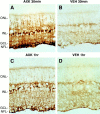
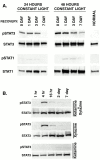
Ciliary neurotrophic factor (CNTF) is pleiotrophic for central, peripheral, and sensory neurons. In the mature retina, CNTF treatment enhances survival of retinal ganglion and photoreceptor cells exposed to otherwise lethal perturbation. To understand its mechanism of action in vivo, the adult rat retina was used as a model to investigate CNTF-mediated activation of Janus kinase/signal transducer and activator of transcription (Jak-STAT) and ras-mitogen activated protein kinase (ras-MAPK). Intravitreal injection of Axokine, an analog of CNTF, phosphorylates STAT3 and MAPK and produces delayed upregulation of total STAT3 and STAT1 protein in rat retina. Activated STAT3 is predominantly localized in nuclei of retinal Müller (glial) cells, ganglion cells, and astrocytes, but not in photoreceptors. Although CNTF alpha-receptor (CNTFRalpha) mRNA and protein are localized predominantly if not exclusively in retinal neurons, coincident CNTF-mediated STAT3 signaling was observed in both glia and neurons. CNTF-induced activation of Jak-STAT signaling prompted us to investigate STAT3 phosphorylation after a variety of stress-mediated, conditioning stimuli. We show that STAT3 is activated in the retina after exposure to subtoxic bright light, mechanical trauma, and systemic administration of the alpha(2)-adrenergic agonist xylazine, all of which have been shown previously to condition photoreceptors to resist light-induced degeneration. These results demonstrate that CNTF directly stimulates Jak-STAT and ras-MAPK cascades in vivo and strongly suggest that STAT3 signaling is an underlying component of neural responsiveness to stress stimuli. The observation that CNTF activates STAT3 in ganglion cells, but not in photoreceptors, suggests that Jak-STAT signaling influences neuronal survival via both direct and indirect modes of action.
Figures



References
-
- Acarin L, Gonzalez B, Castellano B. Stat3 and NFκB glial expression after excitotoxic damage to the postnatal brain. NeuroReport. 1998;9:2869–2873. - PubMed
-
- Adler R, Landa KB, Manthorpe M, Varon S. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979;204:1434–1436. - PubMed
-
- Bonni A, Frank DA, Schindler C, Greenberg ME. Characterization of a pathway for ciliary neurotrophic factor signaling to the nucleus. Science. 1993;262:1575–1579. - PubMed
-
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
